1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY/T 1753-2020 English PDF (YY/T1753-2020)
YY/T 1753-2020 English PDF (YY/T1753-2020)
Regular price
$290.00
Regular price
Sale price
$290.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY/T 1753-2020: Reporting Guide of Unique Device Identification Database
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY/T 1753-2020 (Self-service in 1-minute)
Newer / historical versions: YY/T 1753-2020
Preview True-PDF
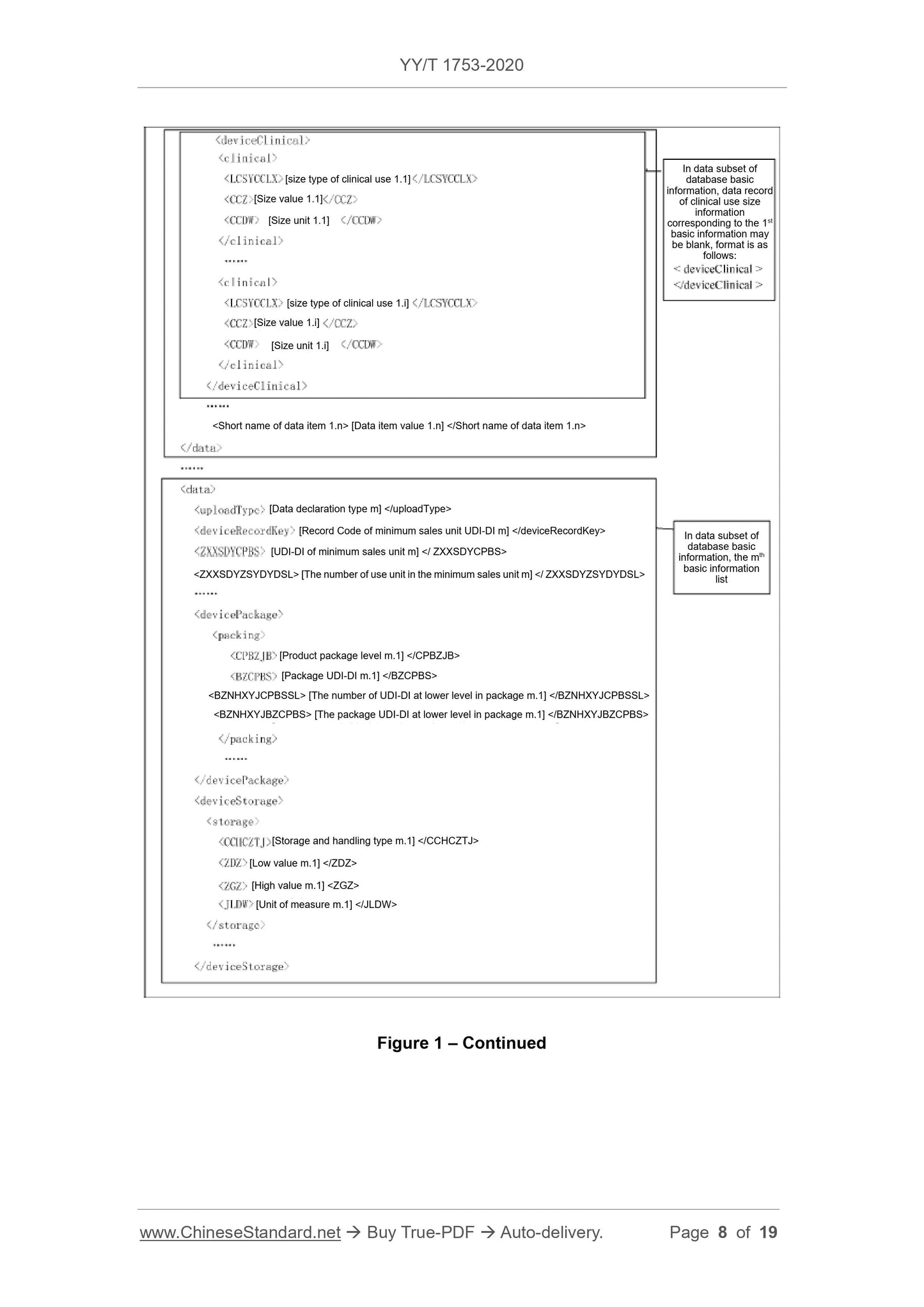
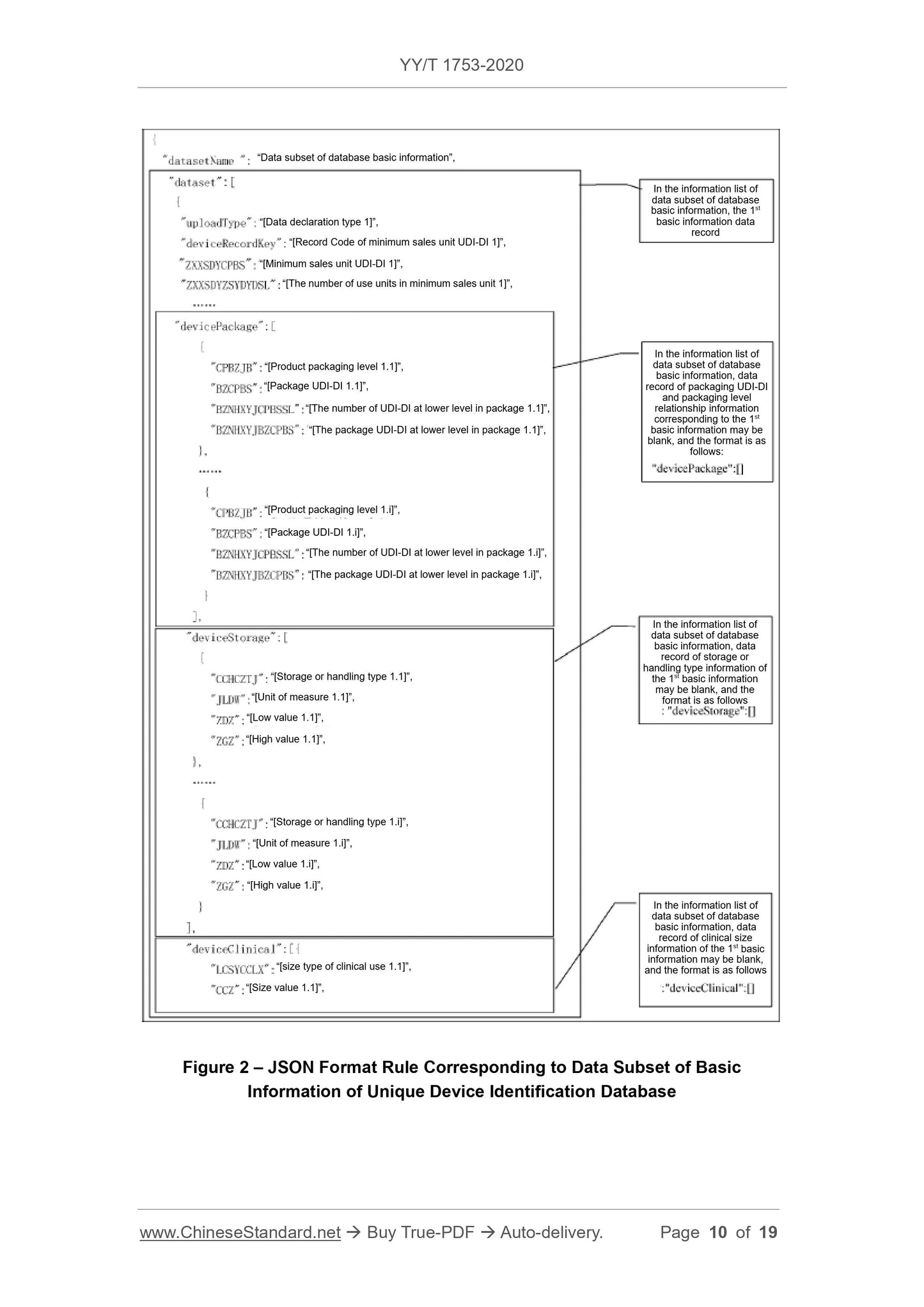
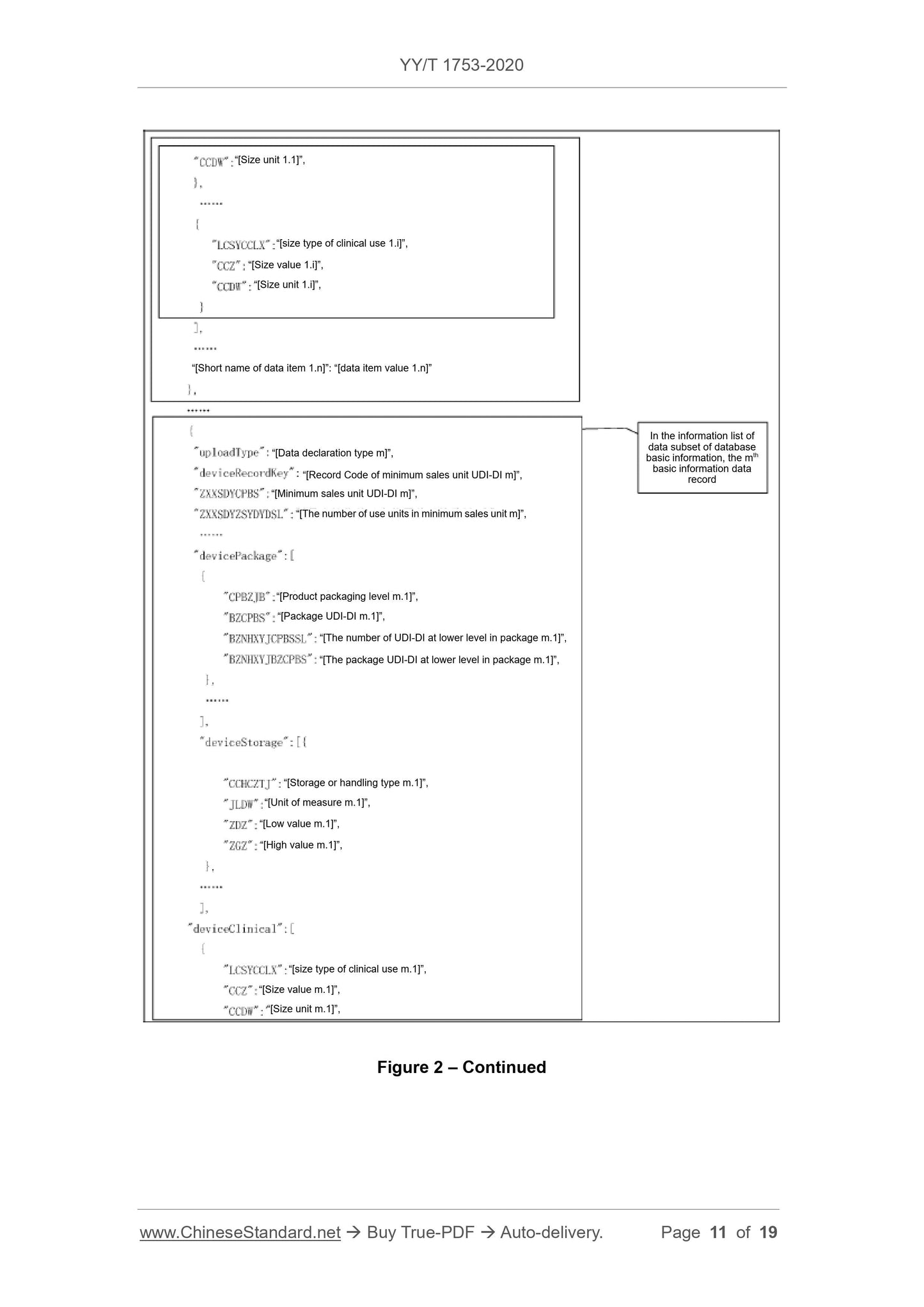
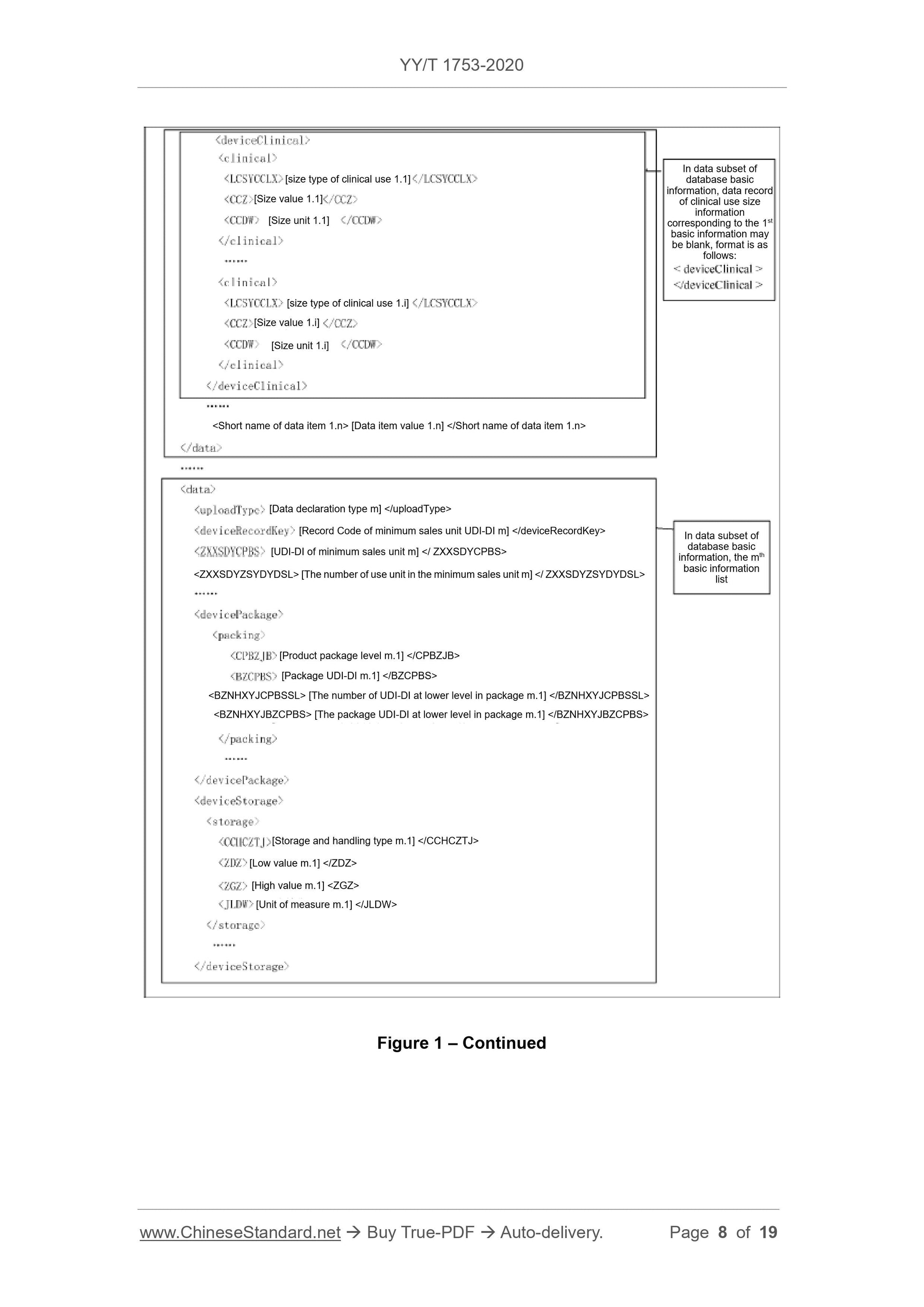
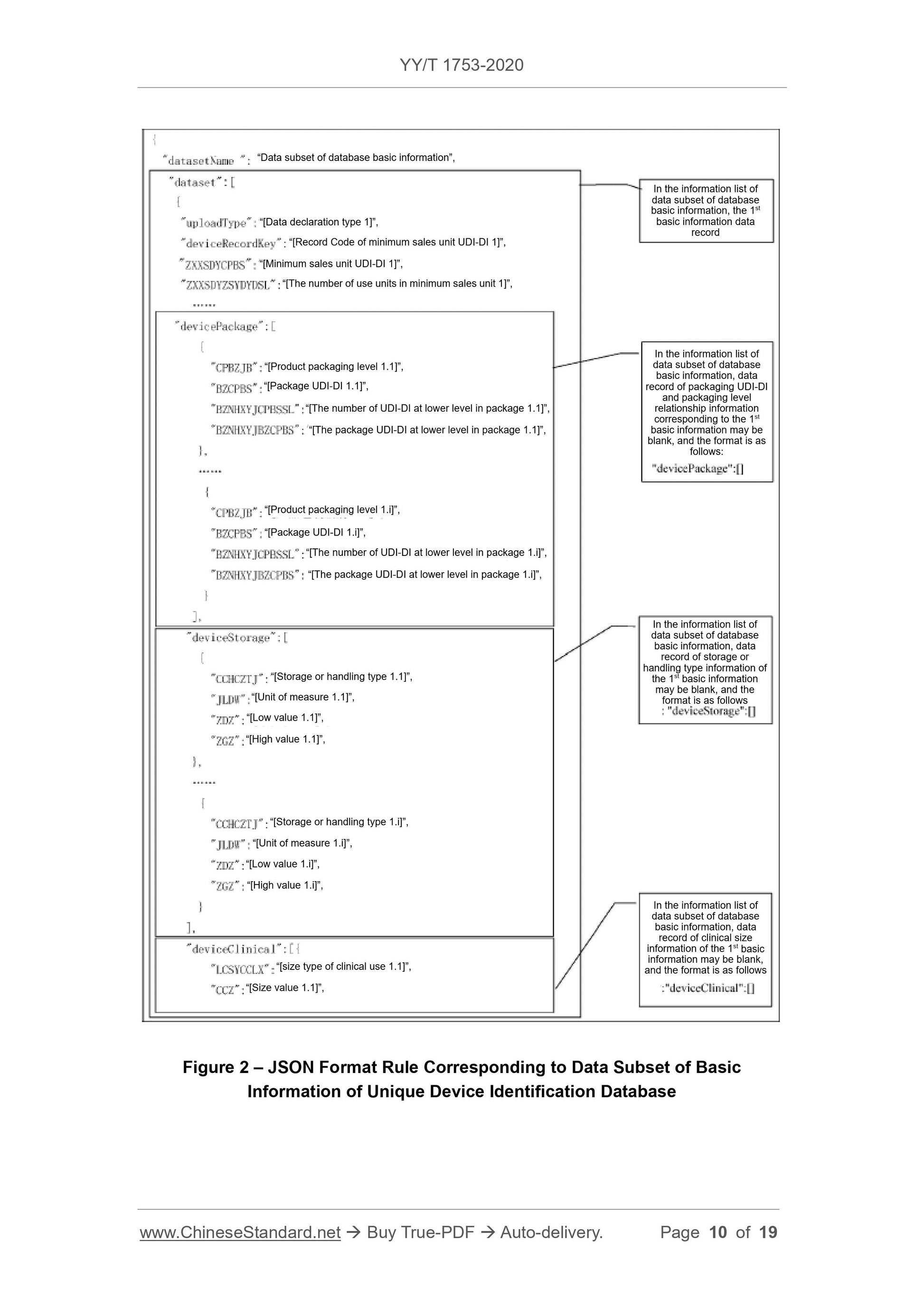
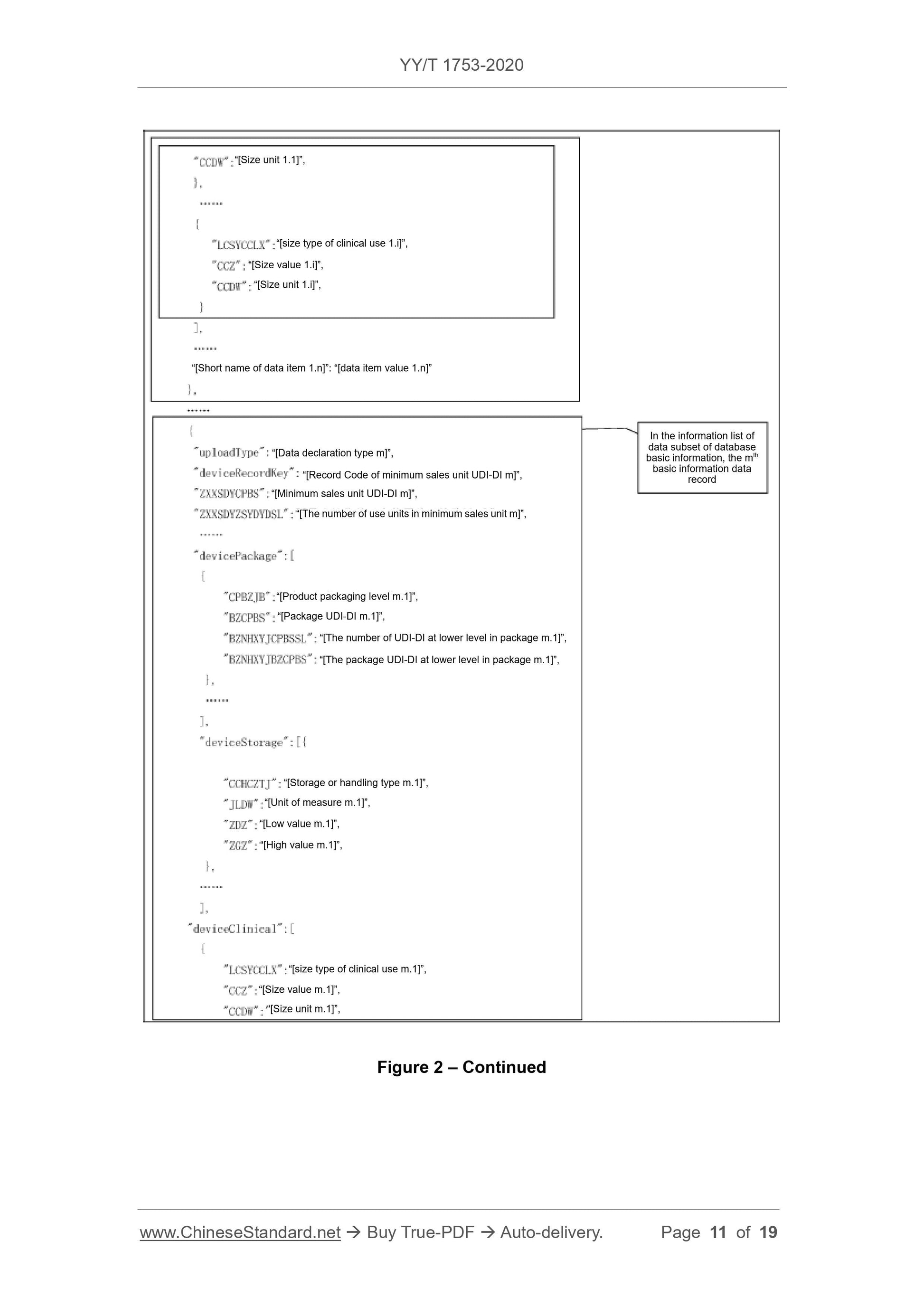
Scope
This Standard specifies the basic requirements for reporting the product identificationand related information to the unique device identification database (referred to
database for short).
This Standard is applicable to the reporting of the unique device identification database.
Basic Data
| Standard ID | YY/T 1753-2020 (YY/T1753-2020) |
| Description (Translated English) | Reporting Guide of Unique Device Identification Database |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard (Recommended) |
| Classification of Chinese Standard | C30 |
| Classification of International Standard | 11.040; 35.240.01 |
| Word Count Estimation | 18,171 |
| Date of Issue | 2020-06-30 |
| Date of Implementation | 2020-10-01 |
| Issuing agency(ies) | State Drug Administration |
Share