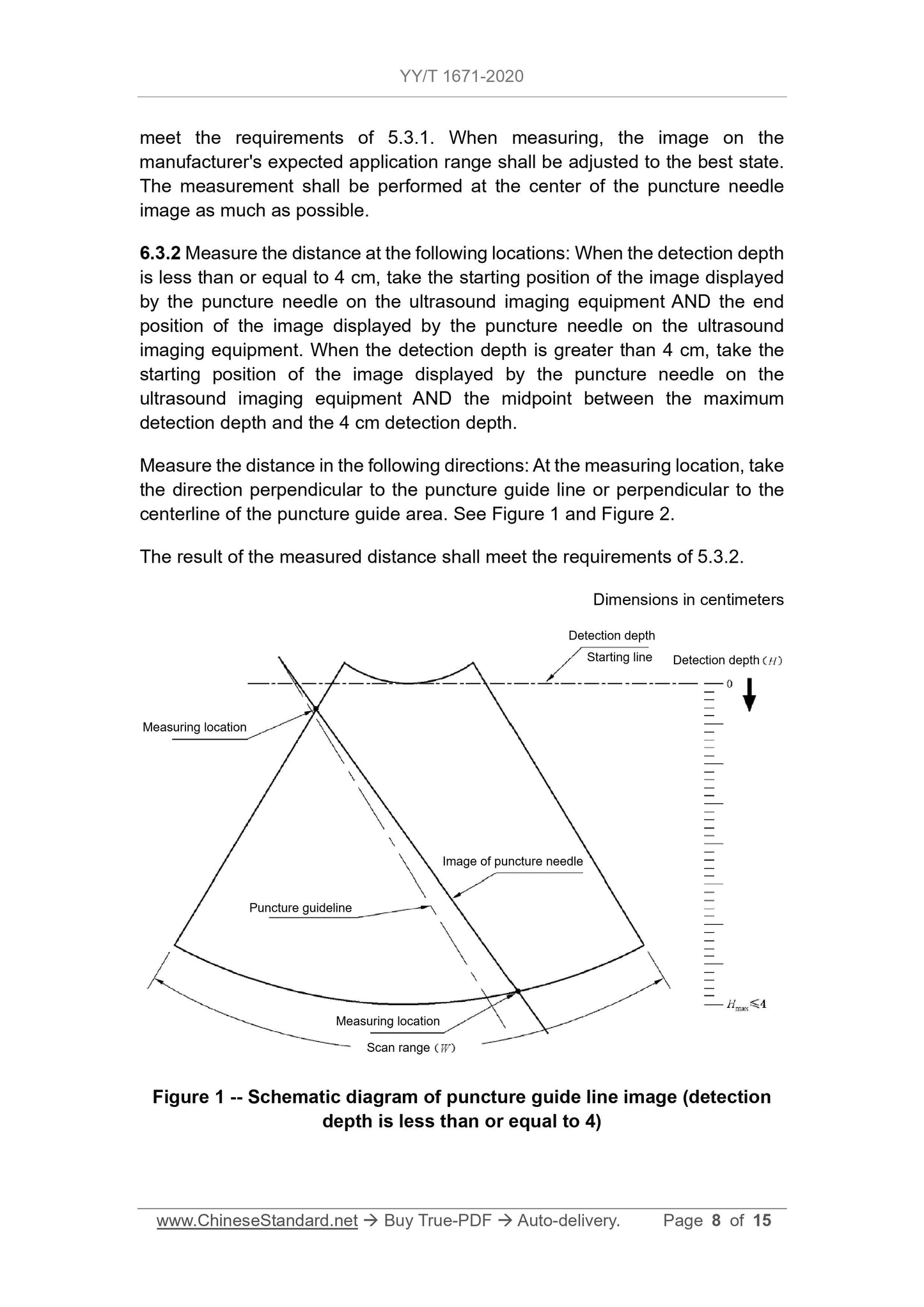
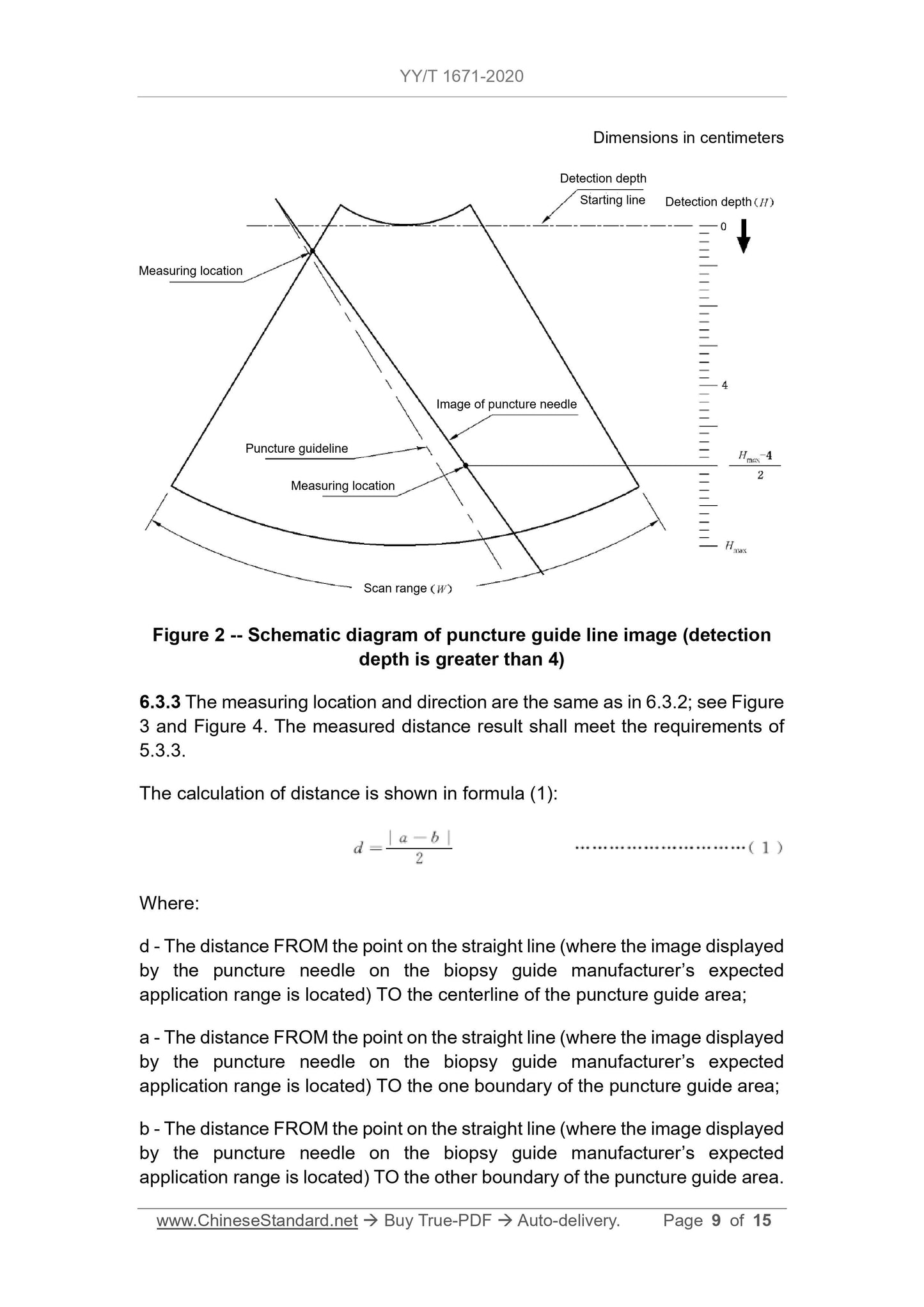
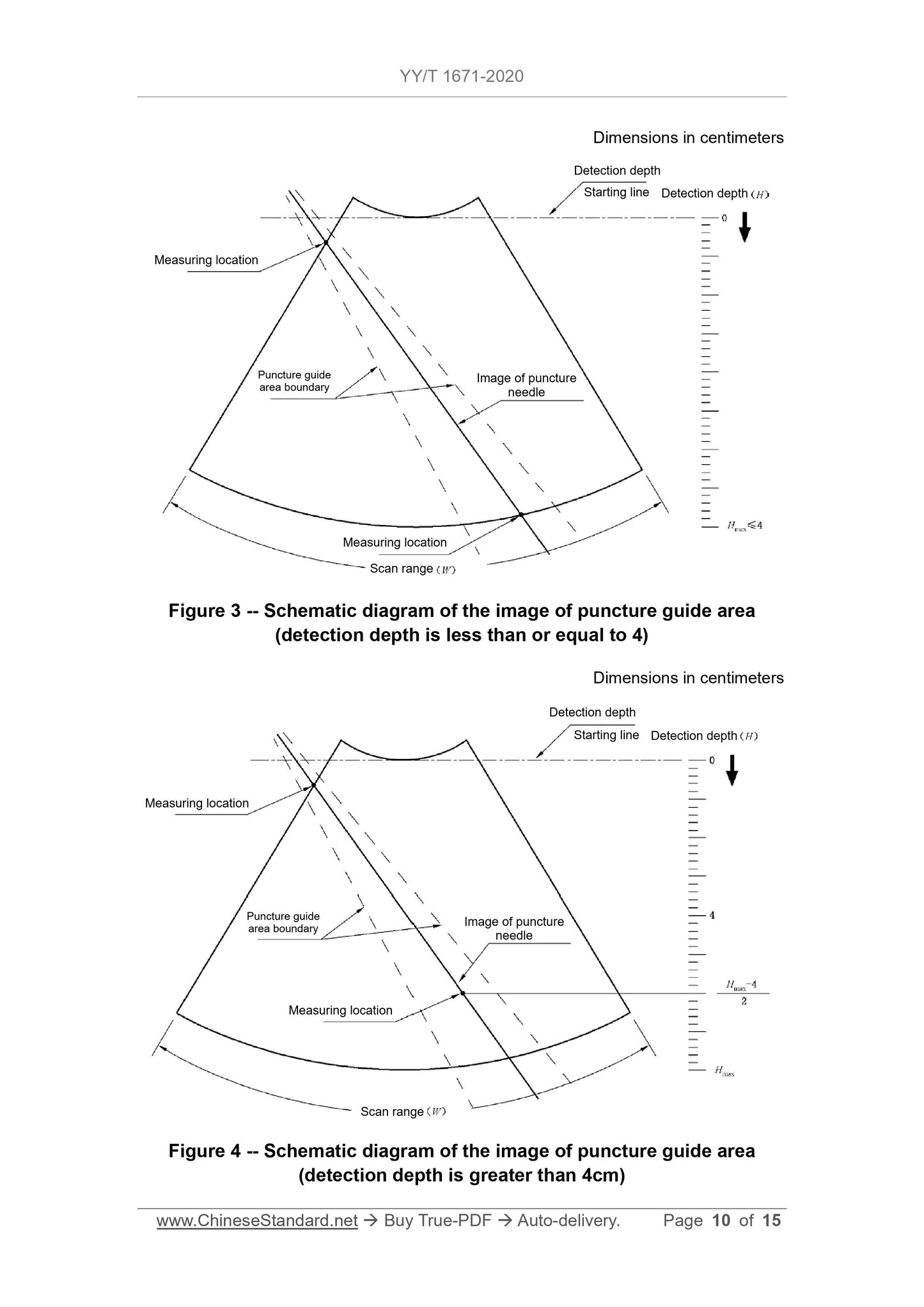
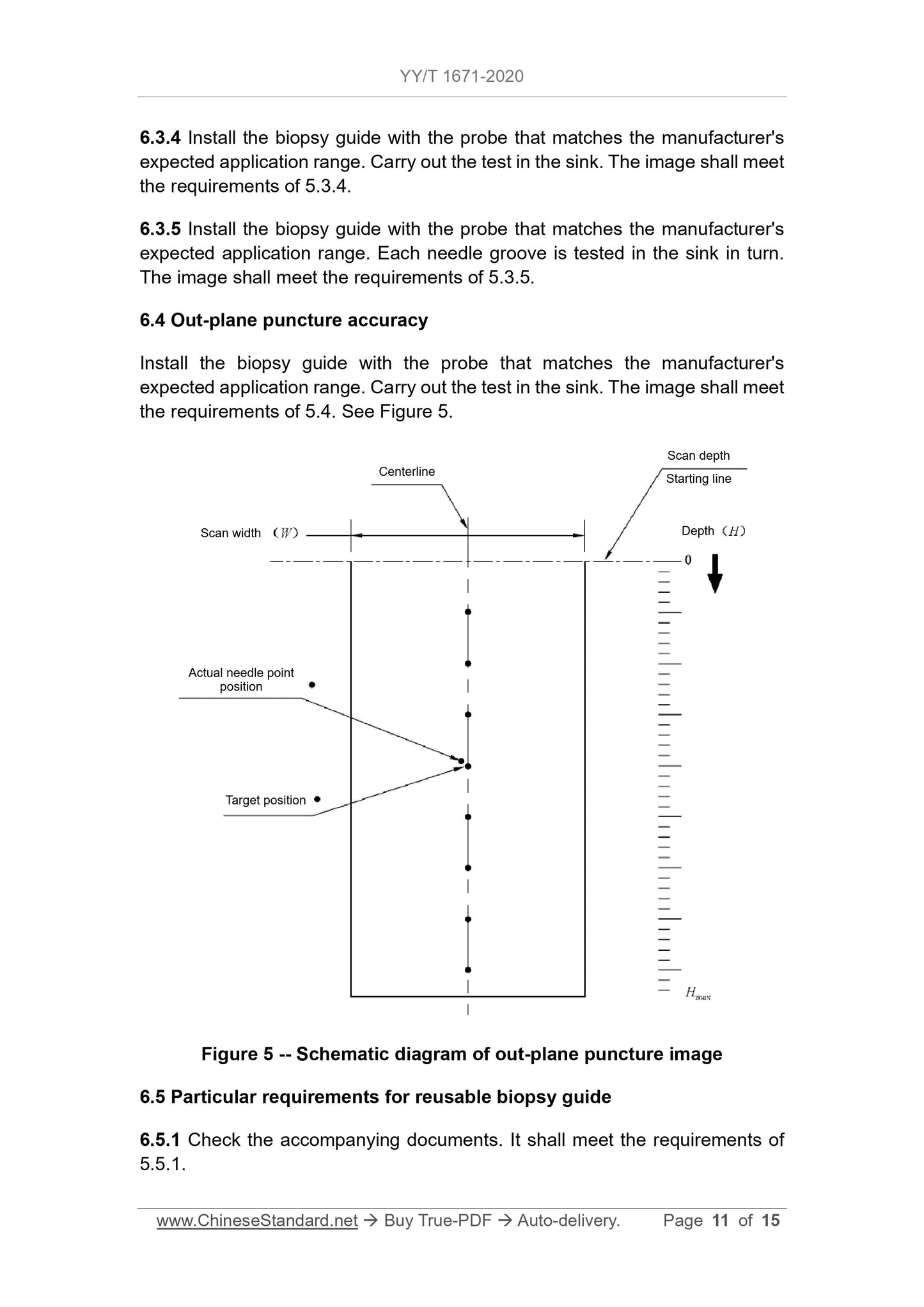
1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY/T 1671-2020 English PDF (YY/T1671-2020)
YY/T 1671-2020 English PDF (YY/T1671-2020)
Regular price
$175.00
Regular price
Sale price
$175.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY/T 1671-2020: Ultrasound biopsy guide
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY/T 1671-2020 (Self-service in 1-minute)
Newer / historical versions: YY/T 1671-2020
Preview True-PDF
Scope
This Standard specifies the terms and definitions, structure type andnomenclature, requirements, and test methods for ultrasound biopsy guide.
This Standard applies to the ultrasound biopsy guide.
This Standard does not apply to inductive navigation devices, such as magnetic
navigation devices.
Basic Data
| Standard ID | YY/T 1671-2020 (YY/T1671-2020) |
| Description (Translated English) | Ultrasound biopsy guide |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard (Recommended) |
| Classification of Chinese Standard | C41 |
| Classification of International Standard | 11.040.50 |
| Word Count Estimation | 11,115 |
| Date of Issue | 2020-02-25 |
| Date of Implementation | 2022-03-01 |
| Issuing agency(ies) | State Drug Administration |
| Summary | This standard specifies the terms and definitions, structural types and nomenclature, requirements and test methods of ultrasonic probe puncture racks. This standard applies to ultrasonic probe puncture racks. This standard does not apply to induction navigation devices, such as magnetic navigation devices. |
Share