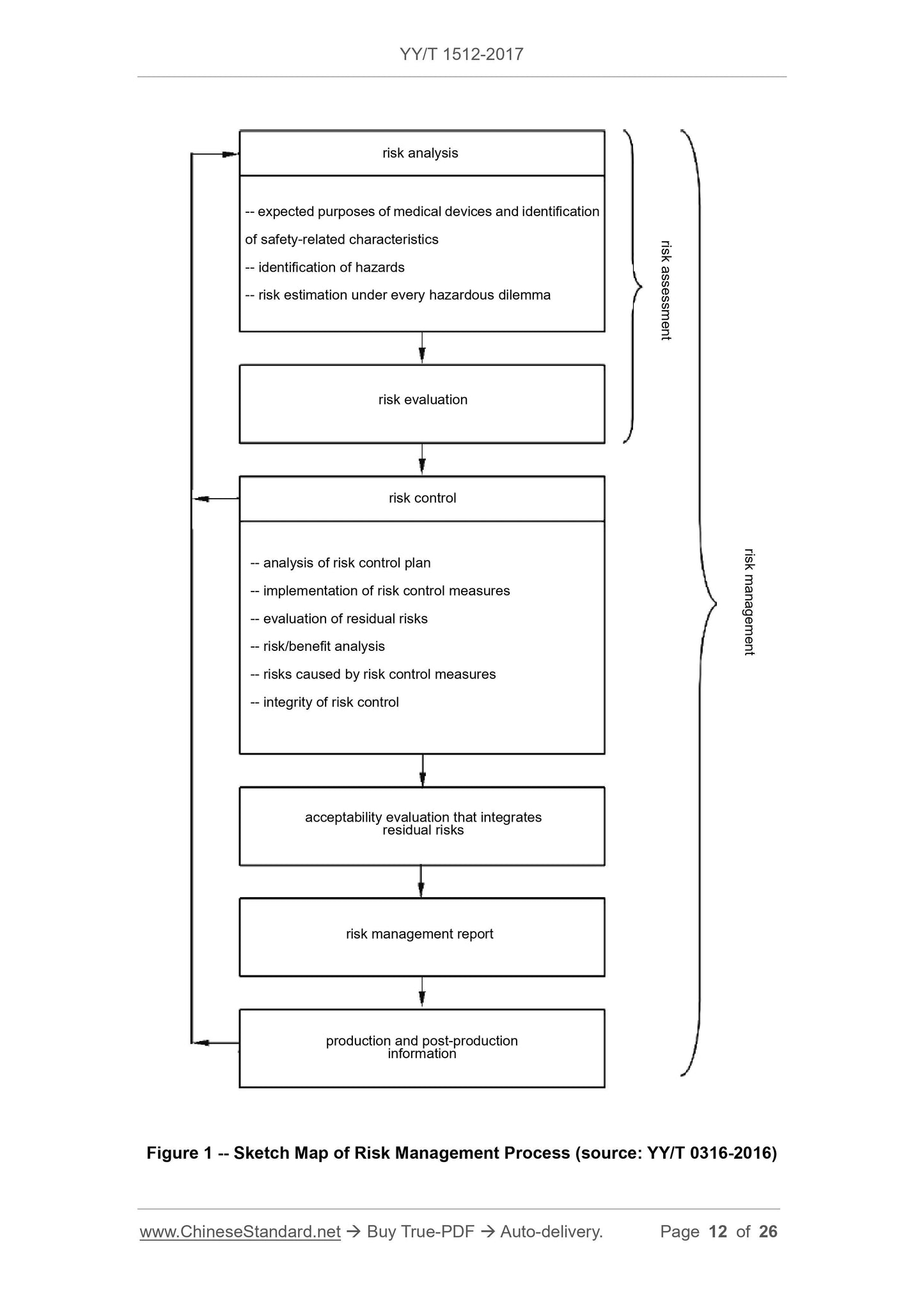
1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY/T 1512-2017 English PDF (YY/T1512-2017)
YY/T 1512-2017 English PDF (YY/T1512-2017)
Regular price
$145.00
Regular price
Sale price
$145.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY/T 1512-2017: Biological evaluation of medical devices - Guidance on the conduct of biological evaluation within a risk management process
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY/T 1512-2017 (Self-service in 1-minute)
Newer / historical versions: YY/T 1512-2017
Preview True-PDF
Scope
This Standard is applicable to the biological evaluation of medical devices inaccordance with the requirements in GB/T 16886.1-2011. This Standard does not add
or modify the requirements in GB/T 16886.1-2011. This Standard does not include
requirements of regulatory inspection or certification and assessment activities.
This Standard is applicable to all the biological evaluation of various types of medical
devices, including active, passive, implantable and non-implantable medical devices.
Basic Data
| Standard ID | YY/T 1512-2017 (YY/T1512-2017) |
| Description (Translated English) | Biological evaluation of medical devices - Guidance on the conduct of biological evaluation within a risk management process |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard (Recommended) |
| Classification of Chinese Standard | C30 |
| Classification of International Standard | 11.040.01 |
| Word Count Estimation | 14,140 |
| Date of Issue | 2017-07-17 |
| Date of Implementation | 2018-07-01 |
| Issuing agency(ies) | State Food and Drug Administration |
Share