1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY/T 0987.1-2016 English PDF (YY/T0987.1-2016)
YY/T 0987.1-2016 English PDF (YY/T0987.1-2016)
Regular price
$140.00
Regular price
Sale price
$140.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY/T 0987.1-2016: Implants for surgery. Magnetic resonance compatibility. Part 1: Safety marking
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY/T 0987.1-2016 (Self-service in 1-minute)
Newer / historical versions: YY/T 0987.1-2016
Preview True-PDF
Scope
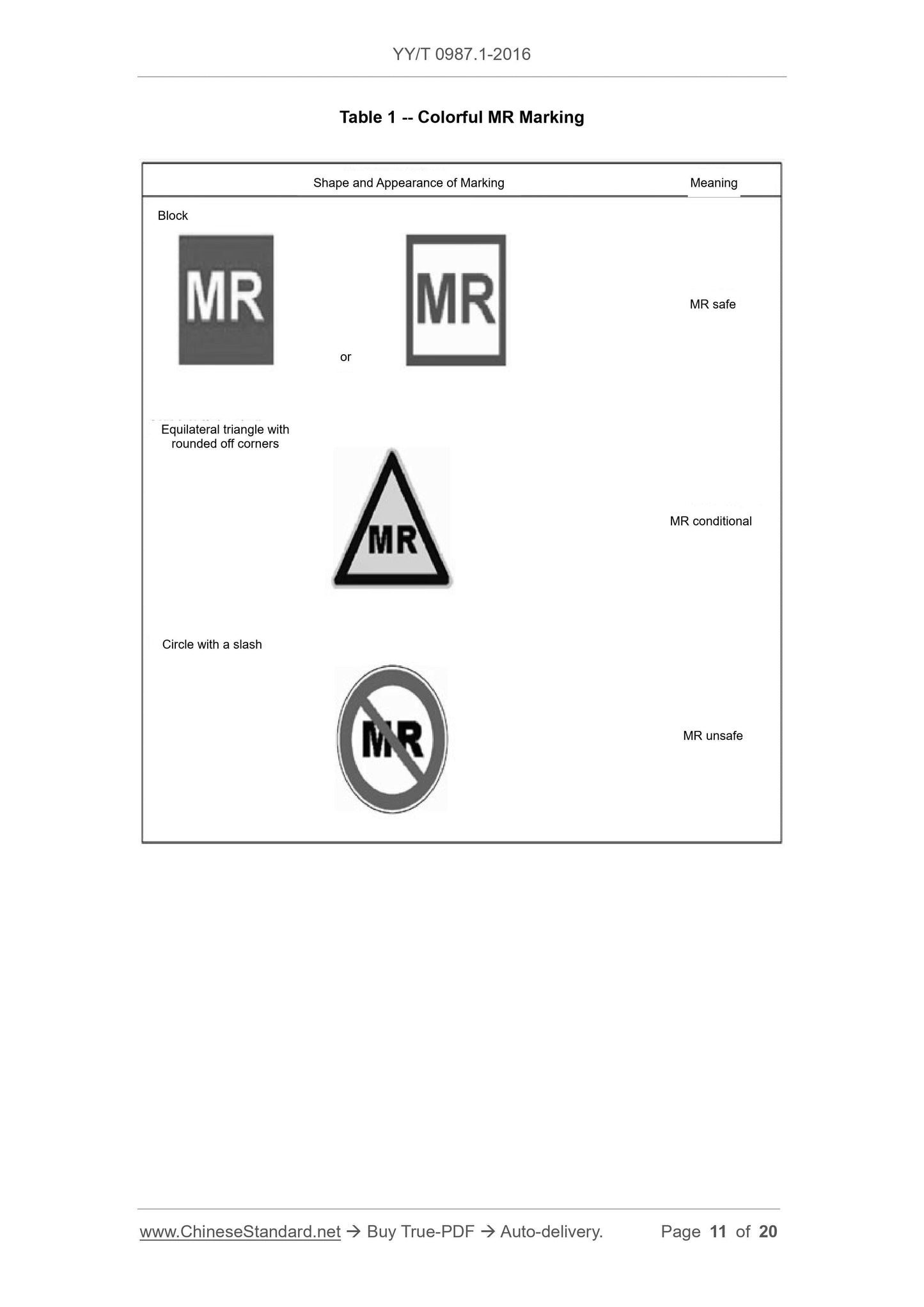
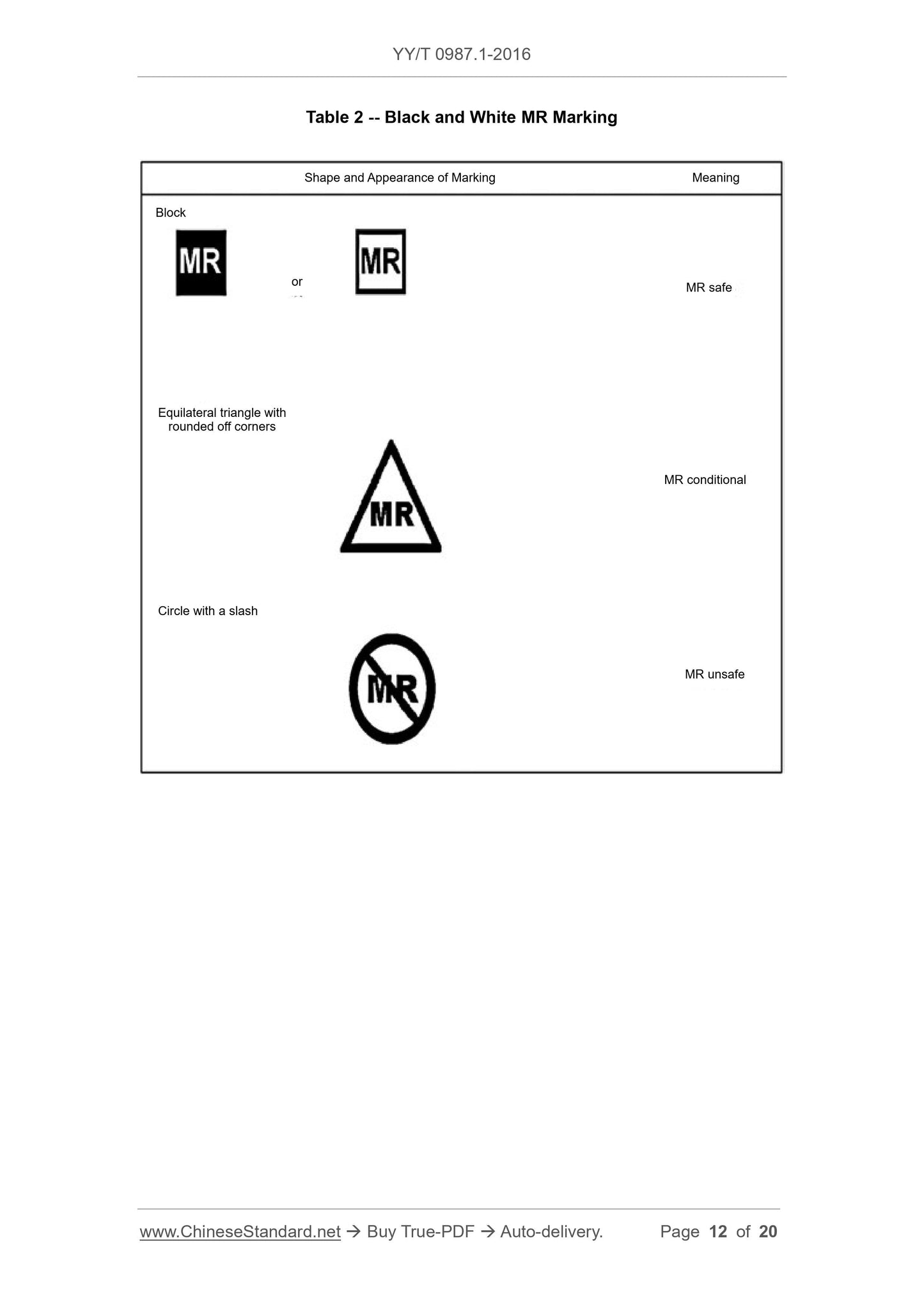
This Part of YY/T 0987 specifies safety marking of medical devices and other items inthe magnetic resonance (MR) environment, so as to provide prompt message on safety.
NOTE 1. other than implants for surgery, other medical devices or items that enter the
magnetic resonance environment can also refer to this Part for safety marking.
NOTE 2. If this Part conflicts with relevant laws and regulations, the laws and regulations
shall prevail.
This Part has the following purposes.
(1) Suggest that an item, that might enter MR environment, should be permanently
marked, so as to indicate whether this item is safe in MR environment;
(2) Suggest the information that shall be included in the marking.
Sometimes it is not realistic to directly mark on implants and certain medical devices.
When it is impossible to directly mark on them, it is suggested to mark on labels and
patients’ information cards.
This Part does not include the content of image artifact, because artifact does not
belong to the issue of safety.
This Part adopts numerical value under international system of units as the standard;
numerical value in the brackets shall merely be considered as reference.
This Part does not attempt to elaborate all the involved safety questions, even though
those safety questions are related with the usage. Determining appropriate safety and
health specifications and clarifying the applicability of management limit before
application is the responsibility on the users of this Standard.
Basic Data
| Standard ID | YY/T 0987.1-2016 (YY/T0987.1-2016) |
| Description (Translated English) | Implants for surgery. Magnetic resonance compatibility. Part 1: Safety marking |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard (Recommended) |
| Classification of Chinese Standard | C35 |
| Classification of International Standard | 11.040.40 |
| Word Count Estimation | 13,185 |
| Date of Issue | 2016-03-23 |
| Date of Implementation | 2017-01-01 |
| Quoted Standard | GB/T 2893.1-2004; YY/T 0987.2; YY/T 0987.3; YY/T 0987.4; YY/T 0987.5 |
| Adopted Standard | ASTM F2503-2008, NEQ |
| Regulation (derived from) | Notice of the General Administration of Food and Drug Administration (No. 74 of 2016) |
| Issuing agency(ies) | State Food and Drug Administration |
| Summary | This standard specifies safety signs for medical devices and other objects in a magnetic resonance (MR) environment to provide safety information. The purpose of this standard is to (1) recommend that objects that may enter the MR environment should be permanently marked to indicate whether the object is safe in the MR environment; and (2) the information to be included in the proposed mark. Direct labeling on implants and certain medical devices is sometimes unrealistic. When not directly marked, it is recommended to mark on the label and patient information card. This standard does not include the artifacts of artifacts because artifacts are not a security issue. This standard adopts the international unit value as the standard, the value in parentheses is for reference only. This standard does not attempt to address all the security issues involved, even if it is related to the use of security issues |
Share