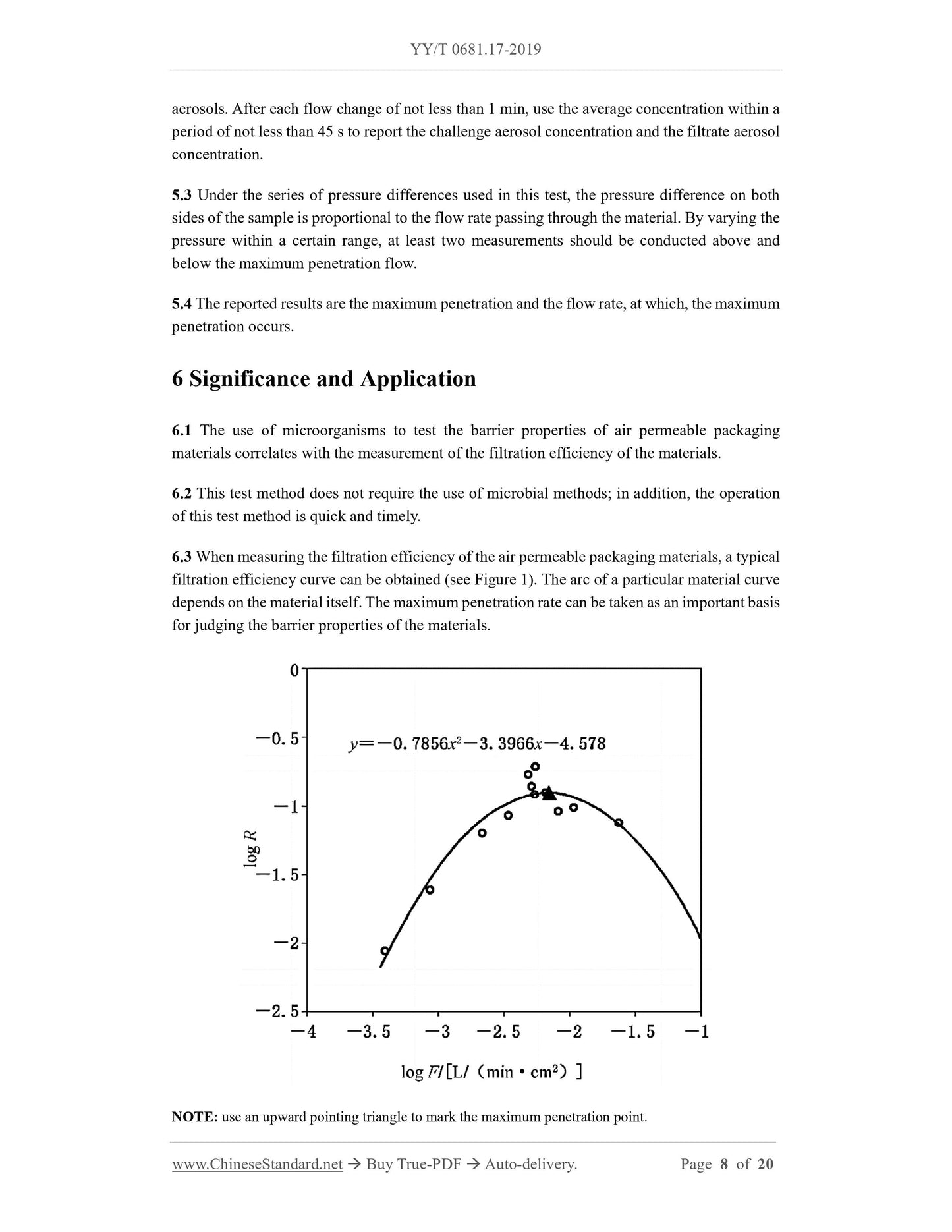
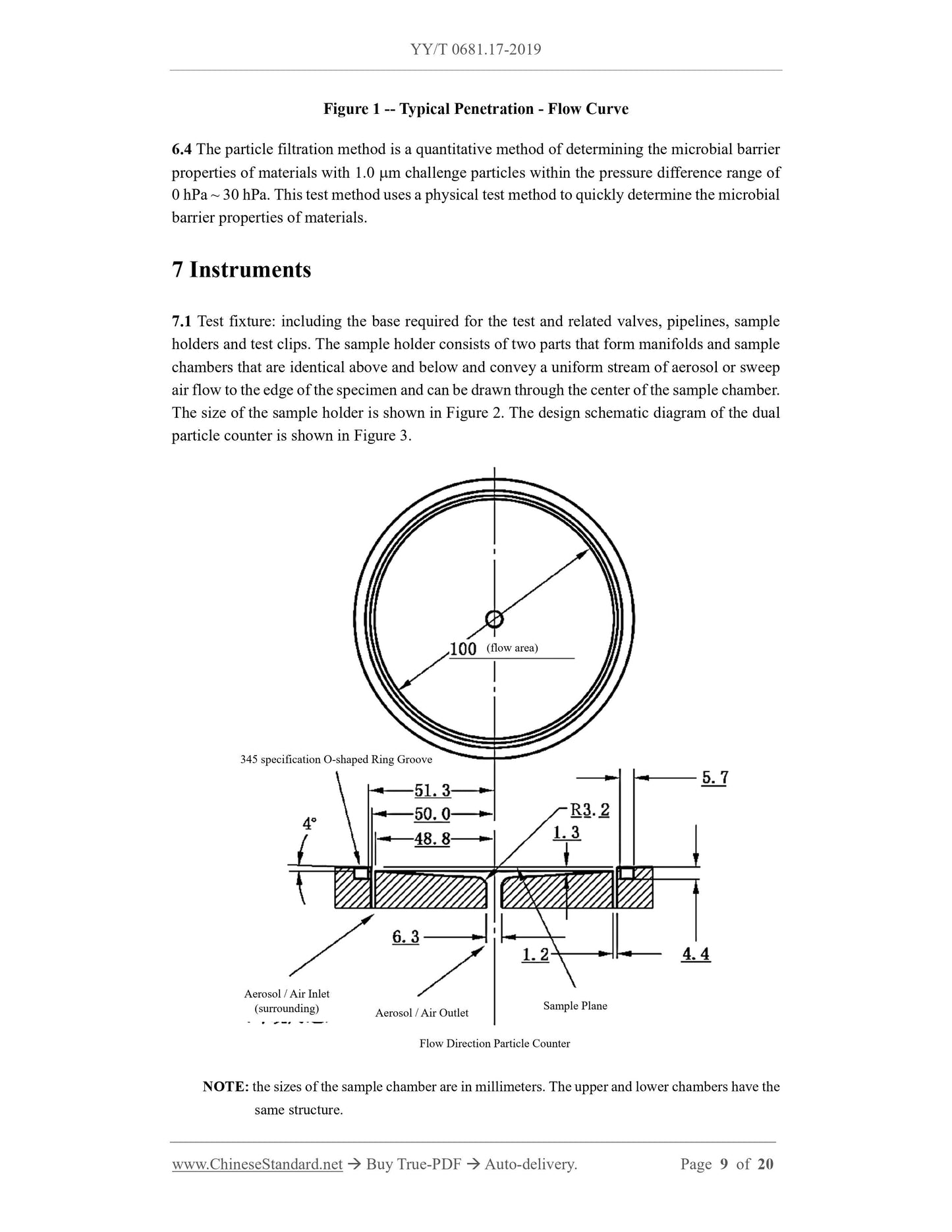
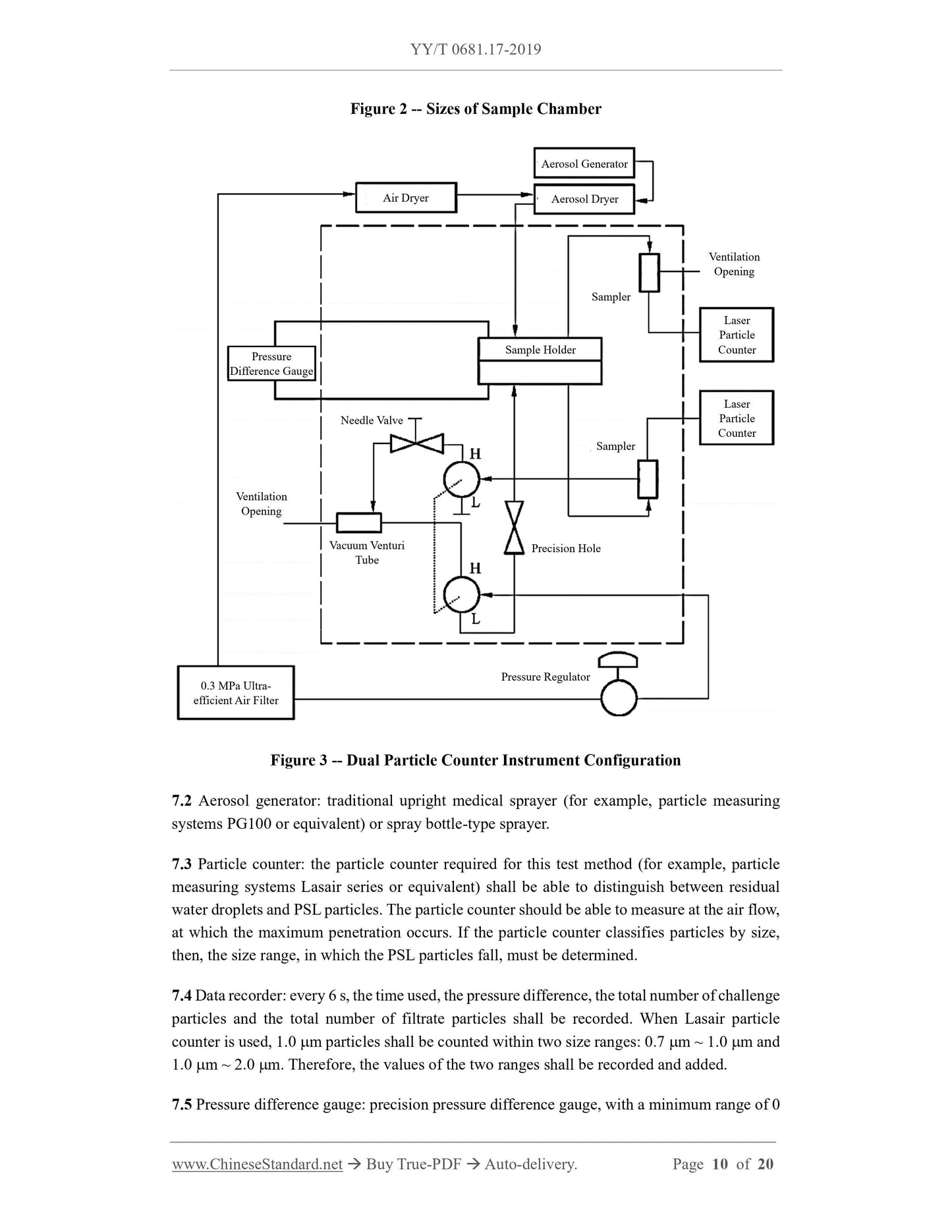
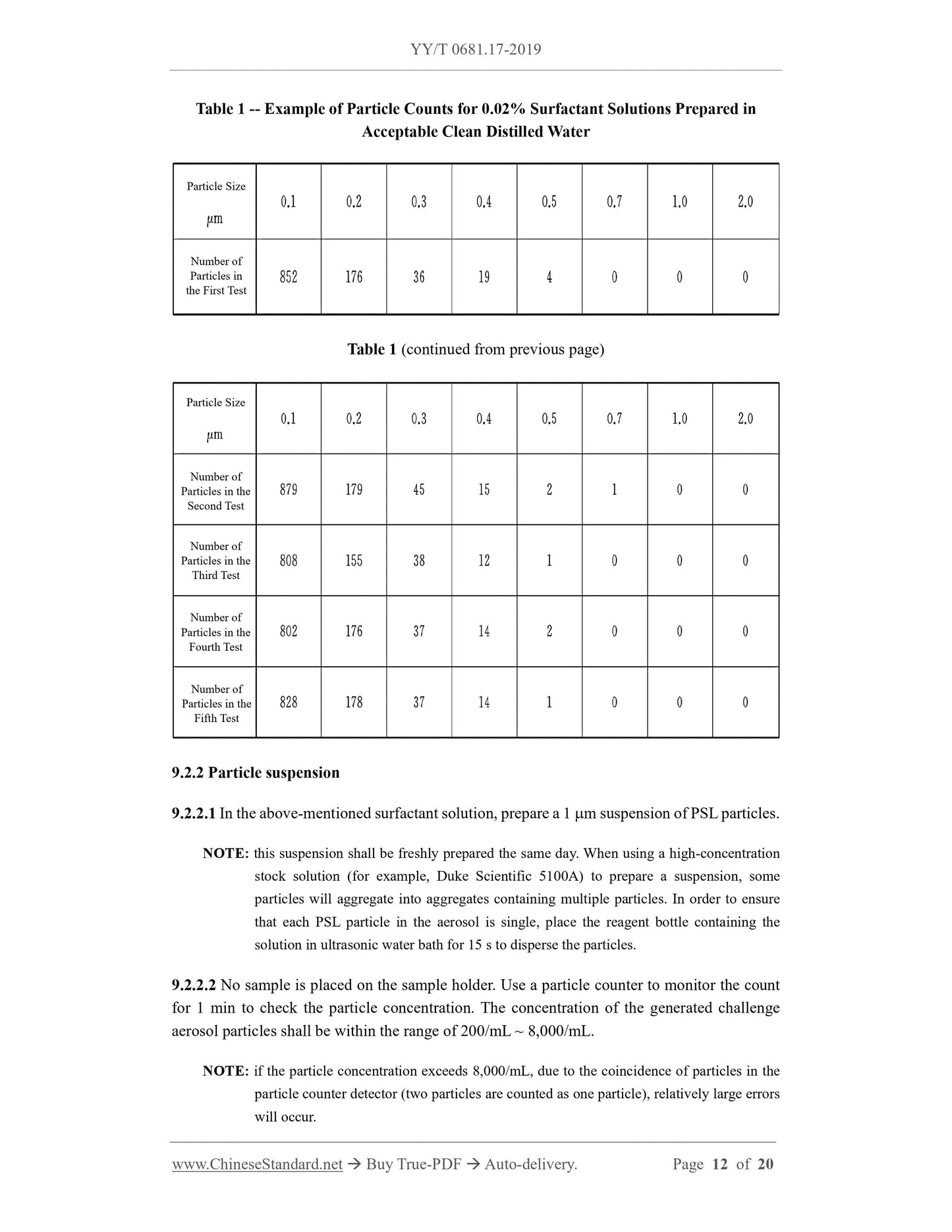
1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY/T 0681.17-2019 English PDF (YY/T0681.17-2019)
YY/T 0681.17-2019 English PDF (YY/T0681.17-2019)
Regular price
$210.00
Regular price
Sale price
$210.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY/T 0681.17-2019: Test methods for sterile medical device package - Part 17: Testing the microbial barrier performance of porous package materials using aerosol filtration method
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY/T 0681.17-2019 (Self-service in 1-minute)
Newer / historical versions: YY/T 0681.17-2019
Preview True-PDF
Scope
This Part of YY/T 0681 specifies the determination of the aerosol filtration performance usingaerosol that generates particles with a diameter of 1.0 m, and the evaluation of the filtration
efficiency of the material using two particle counters.
This Part of YY/T 0681 is applicable to air permeable materials used for the packaging of
terminally sterilized medical devices.
This Part of YY/T 0681 does not apply to materials with Bendtsen air permeability1) over 4,000
mL/min.
Basic Data
| Standard ID | YY/T 0681.17-2019 (YY/T0681.17-2019) |
| Description (Translated English) | Test methods for sterile medical device package - Part 17: Testing the microbial barrier performance of porous package materials using aerosol filtration method |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard (Recommended) |
| Classification of Chinese Standard | C31 |
| Classification of International Standard | 11.080.40 |
| Word Count Estimation | 12,167 |
| Date of Issue | 2019 |
| Date of Implementation | 2020-10-01 |
| Issuing agency(ies) | State Drug Administration |
| Summary | This standard specifies the determination of the aerosol filtration performance of breathable packaging materials by generating aerosols of particles with a diameter of 1.0 ��m, and the use of two particle counters to evaluate the filtration efficiency of the material. This standard applies to breathable materials for terminally sterilized medical device packaging. This standard does not apply to materials with a Bentsen air permeability exceeding 4000 mL/min. |
Share