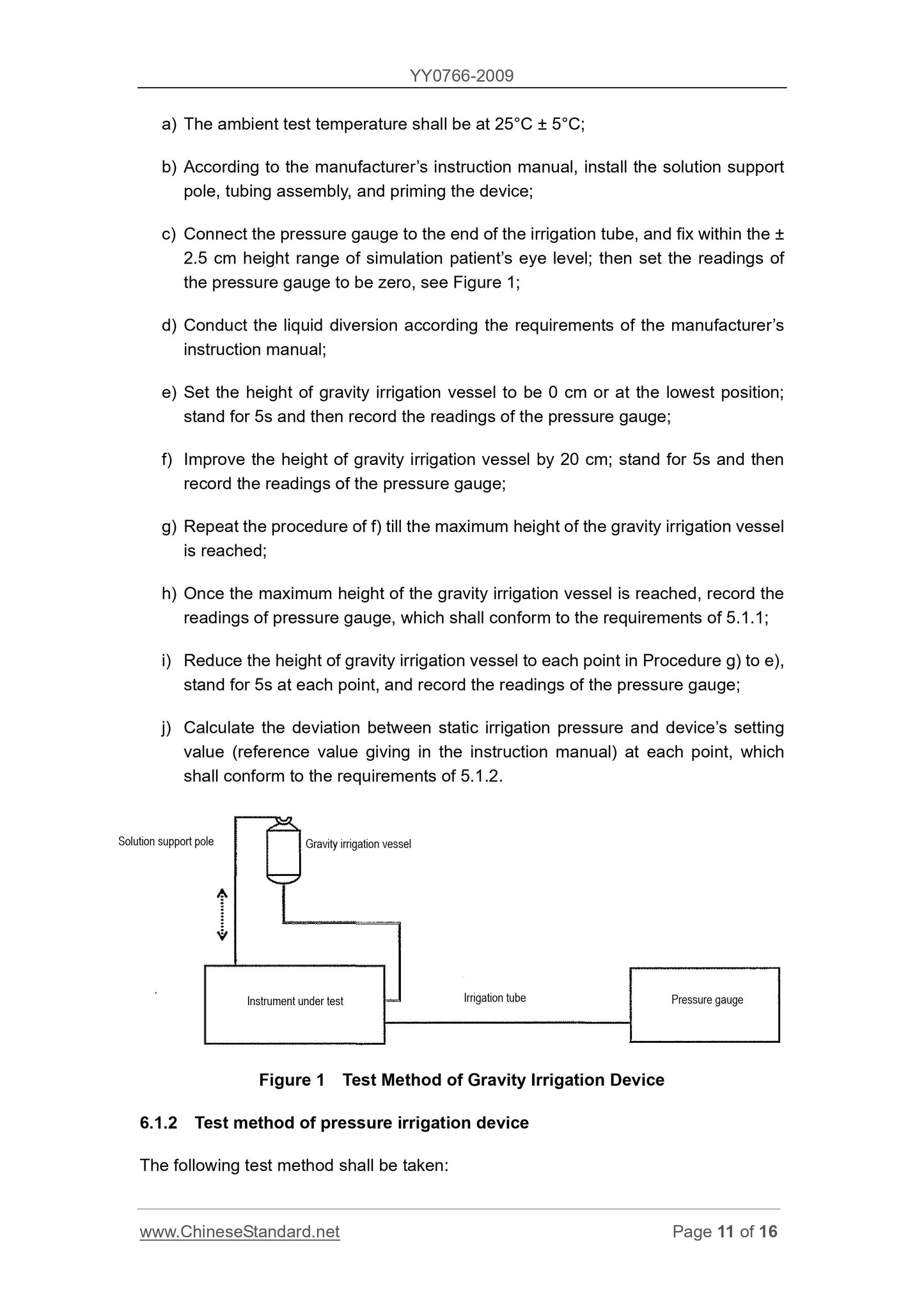
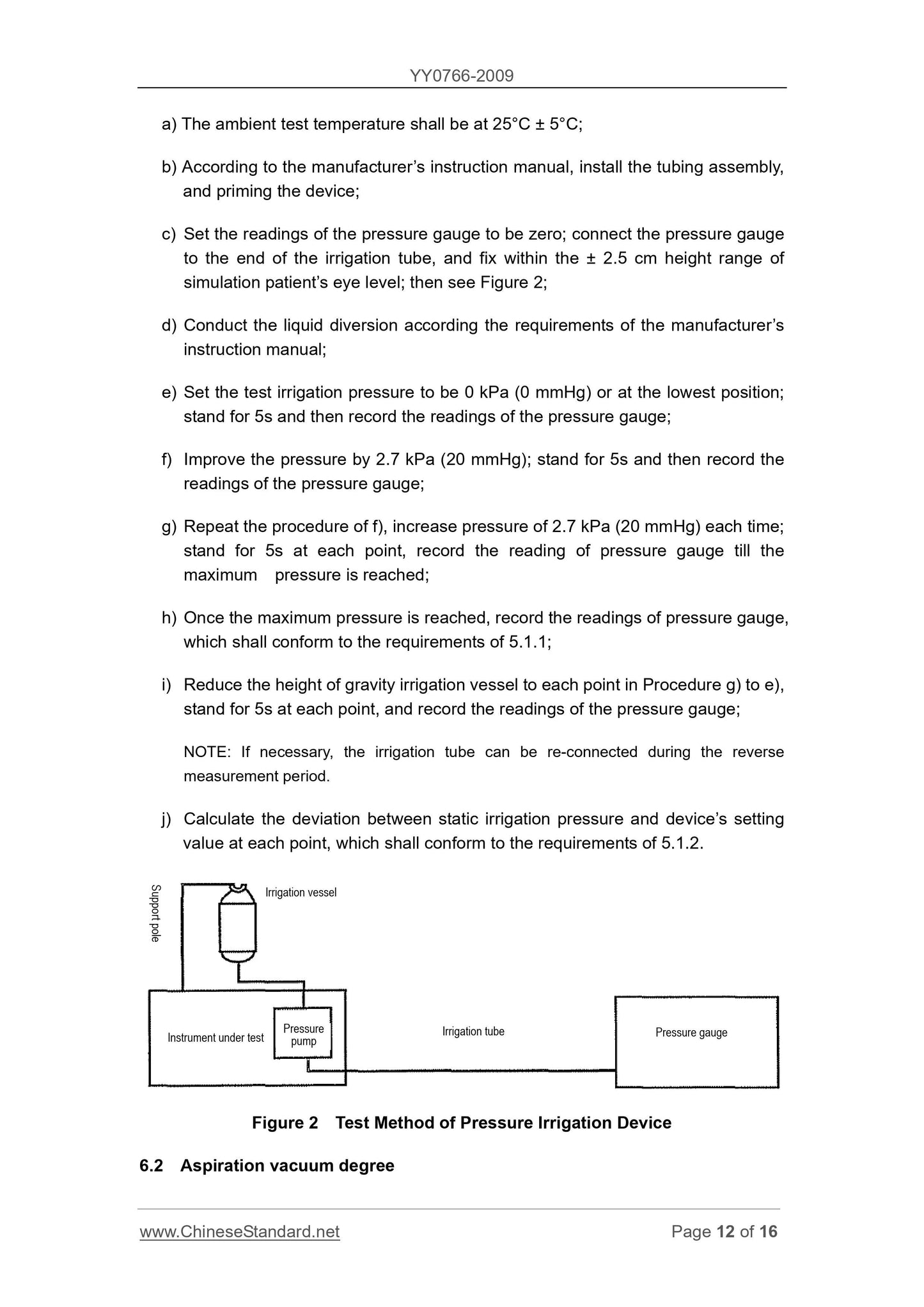
1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY 0766-2009 English PDF
YY 0766-2009 English PDF
Regular price
$140.00
Regular price
Sale price
$140.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY 0766-2009: Lens ultrasonic removal and vitrectomy devices for ophthalmic surgery
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY 0766-2009 (Self-service in 1-minute)
Newer / historical versions: YY 0766-2009
Preview True-PDF
Scope
This Standard specifies the terms and definitions, product classification, requirementsand test methods of lens ultrasonic removal and vitrectomy device for ophthalmic
surgery.
This Standard is applicable to the lens ultrasonic removal device for ophthalmic
surgery (hereinafter refers to device), which also possesses the vitrectomy function.
NOTE. Phacofragmentation of lens indicates, in the earliest period, the surgery to use
ultrasonic energy to crush (or emulsify) cataract lens, and extract lens tissues through small
incision; currently, ultrasound is still the most important means for the lens removal and
vitrectomy device for ophthalmic surgery; recently, there are the device using other energy and
extracting cataract lens through small incision (such as laser and liquefaction); for which this
Standard can be referred to.
Basic Data
| Standard ID | YY 0766-2009 (YY0766-2009) |
| Description (Translated English) | Lens ultrasonic removal and vitrectomy devices for ophthalmic surgery |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard |
| Classification of Chinese Standard | C41 |
| Classification of International Standard | 11.040.70 |
| Word Count Estimation | 11,111 |
| Date of Issue | 2009-12-30 |
| Date of Implementation | 2011-06-01 |
| Quoted Standard | GB 9706.1; GB 9706.15; YY/T 0644-2008; ISO 15752-2000 |
| Issuing agency(ies) | State Food and Drug Administration |
| Summary | This standard specifies the ophthalmic lens removal and vitrectomy equipment ultrasound terminology and definitions, product classification, requirements and test methods. This standard applies to the application of ultrasonic energy to perform eye surgery, cataract extraction equipment (hereinafter referred to as equipment), such devices generally function along with vitrectomy. |
Share