1
/
of
10
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY 0719.5-2009 English PDF
YY 0719.5-2009 English PDF
Regular price
$155.00
Regular price
Sale price
$155.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY 0719.5-2009: Ophthalmic optics. Contact lens care products. Part 5: Determination of physical compatibility of contact lens care products with contact lenses [YY/T 0719.5-2009]
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY 0719.5-2009 (Self-service in 1-minute)
Newer / historical versions: YY 0719.5-2009
Preview True-PDF
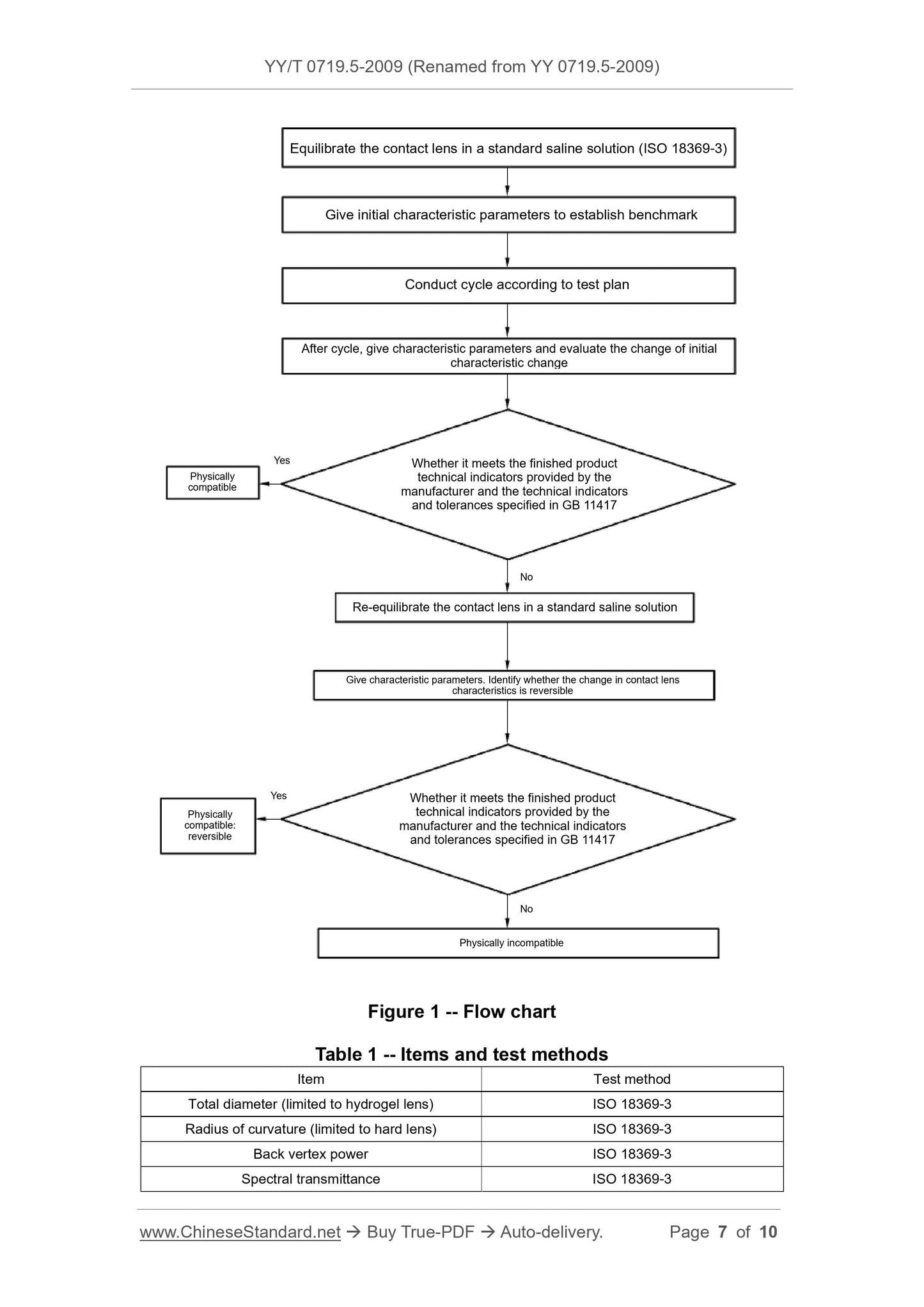
Scope
This Part of YY 0719 provides general steps to evaluate the physicalcompatibility of contact lenses and contact lens care products AND
performance requirements as well as determines whether the observed lens
changes are reversible.
Basic Data
| Standard ID | YY 0719.5-2009 (YY0719.5-2009) |
| Description (Translated English) | Ophthalmic optics. Contact lens care products. Part 5: Determination of physical compatibility of contact lens care products with contact lenses [YY/T 0719.5-2009] |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard |
| Classification of Chinese Standard | C40 |
| Classification of International Standard | 11.040.70 |
| Word Count Estimation | 8,887 |
| Date of Issue | 2009-06-16 |
| Date of Implementation | 2010-12-01 |
| Quoted Standard | YY 0719.1-2009; ISO 18369-2-2006; ISO 18369-3-2006 |
| Adopted Standard | ISO 11981-1999 + COR.1-2005, MOD |
| Regulation (derived from) | Industry standard filing Notice 2009 No. 9 |
| Issuing agency(ies) | State Food and Drug Administration |
| Summary | This standard specifies the evaluation of contact lenses and contact lens care products, general steps physical compatibility and performance requirements, and determine whether the changes observed in the lens reversible. |
Share