1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0585.4-2009 English PDF
YY 0585.4-2009 English PDF
Regular price
$110.00
Regular price
Sale price
$110.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY 0585.4-2009: Fluid lines for use with pressure infusion equipment and accessories for single use - Part 4: Check valves
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY 0585.4-2009 (Self-service in 1-minute)
Newer / historical versions: YY 0585.4-2009
Preview True-PDF
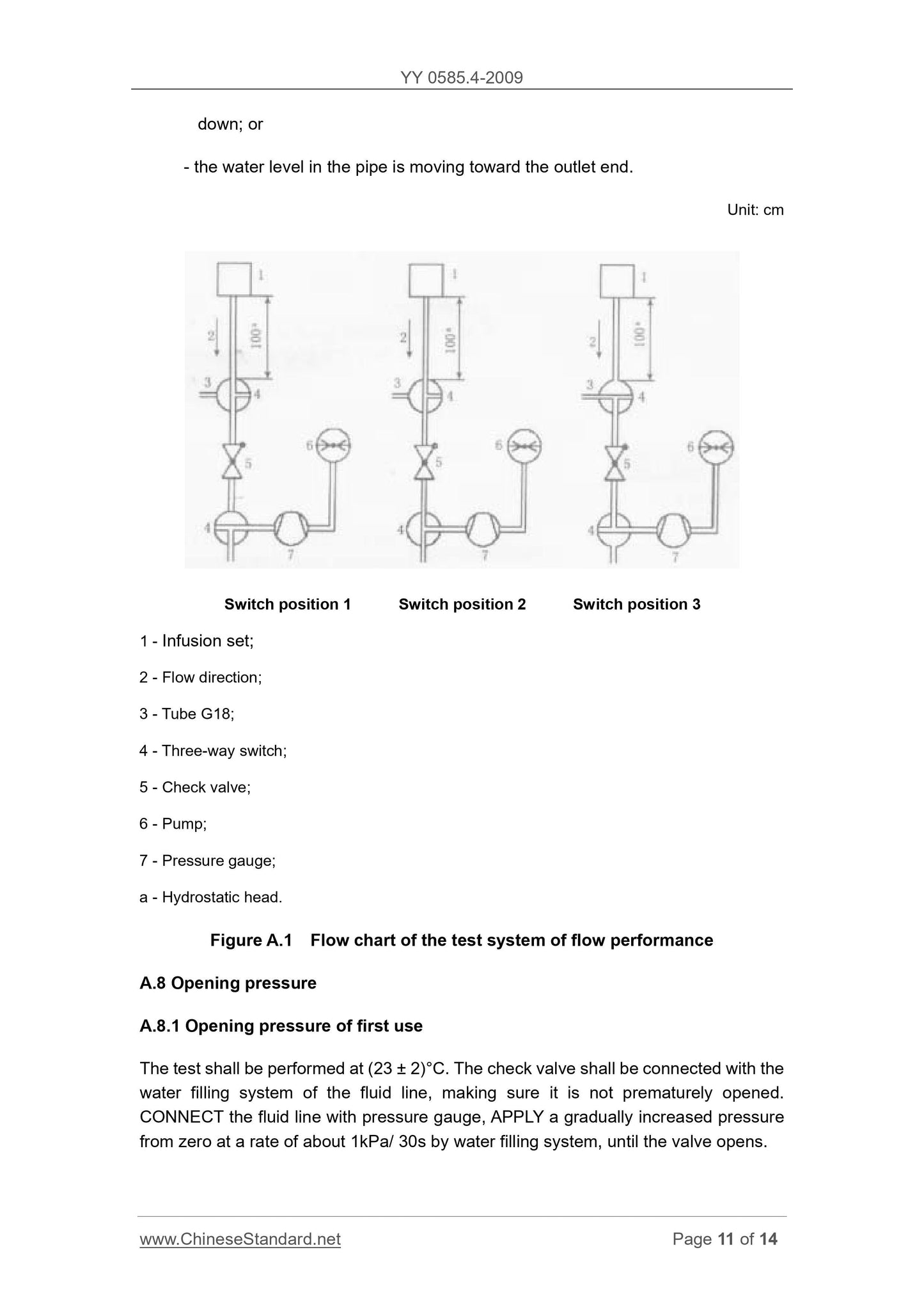
Scope
This Part of YY 0585.4 applies to sterile check valves for gravity feed infusion setsand/or pressure feed infusion sets for single use.
Note. The basic requirements in this Part also applies to the built-in check valves.
Basic Data
| Standard ID | YY 0585.4-2009 (YY0585.4-2009) |
| Description (Translated English) | Fluid lines for use with pressure infusion equipment and accessories for single use - Part 4: Check valves |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard |
| Classification of Chinese Standard | C31 |
| Classification of International Standard | 11.040.20 |
| Word Count Estimation | 9,928 |
| Date of Issue | 2009-12-30 |
| Date of Implementation | 2011-06-01 |
| Quoted Standard | GB/T 1962.2; GB 8368; GB/T 16886.1; YY 0466; ISO 8871-1; ISO 8871-2 |
| Adopted Standard | ISO 8536-12-2007, MOD |
| Issuing agency(ies) | State Food and Drug Administration |
| Summary | This standard applies to single use, gravity infusion and/or pressure with a sterile infusion fluid type backflow preventer. |
Share