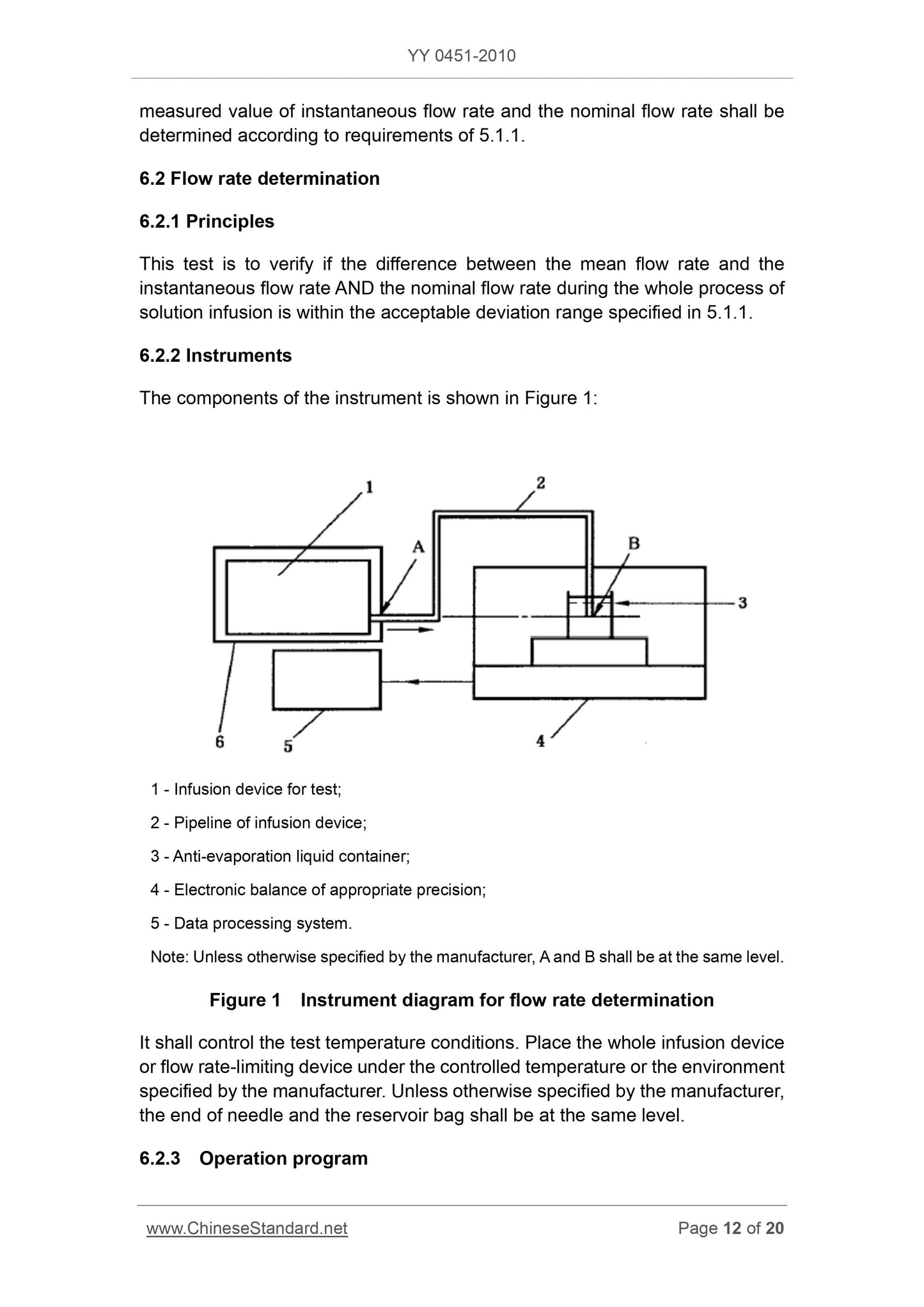
1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0451-2010 English PDF
YY 0451-2010 English PDF
Regular price
$150.00
Regular price
Sale price
$150.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY 0451-2010: Portable infusion devices for single use - Non electrically driven
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY 0451-2010 (Self-service in 1-minute)
Newer / historical versions: YY 0451-2010
Preview True-PDF
Scope
This Standard specifies the basic requirements and appropriate test methodsfor non electrically driven portable infusion devices (hereinafter referred to as
"infusion device"). It is applicable to sustainable infusion device (fixed or
adjustable) and (or) automatic bolus infusion device.
This Standard is not applicable to.
- Electrically driven or electrically controlled infusion devices included by
IEC 60601-2-24;
- Implantable devices;
- Enteral supply pump;
- Transdermal infusion device;
- Device of which the infusion power is not provided by the device but by
the active intervention of patient (e.g. the device which only takes gravity
as power).
Basic Data
| Standard ID | YY 0451-2010 (YY0451-2010) |
| Description (Translated English) | Portable infusion devices for single use - Non electrically driven |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard |
| Classification of Chinese Standard | C46 |
| Classification of International Standard | 11.120.30 |
| Word Count Estimation | 14,125 |
| Date of Issue | 2010-12-27 |
| Date of Implementation | 2012-06-01 |
| Older Standard (superseded by this standard) | YY 0451-2003 |
| Quoted Standard | GB/T 1962.1; GB/T 1962.2; GB 8368; GB/T 14233.1; GB/T 14233.2; GB/T 16886.1; GB/T 16886.2; GB/T 16886.3; GB/T 16886.4; GB/T 16886.5; GB/T 16886.6; GB/T 16886.7; GB/T 16886.9; GB/T 16886.10; GB/T 16886.11; GB/T 16886.12; GB/T 16886.13; GB/T 16886.14; GB/T 16886.15; GB/T 16886.16; GB/T 16886.17; GB/T 6682; YY/T 0466.1 |
| Regulation (derived from) | State Food and Drug Administration Notice 2010 No. 97 |
| Issuing agency(ies) | State Food and Drug Administration |
| Summary | This standard specifies the use of non- electrically driven portable infusion pump disposable basic requirements and the corresponding test methods. For sustainable solution to (fixed or adjustable), and (or) controlled infusion pump for liquid. This standard does not apply to: IEC 60601-2-24 included electric drive or electric controlled infusion pump, implantable devices, intestinal supplies pumps, liquid transdermal device, infusion powered power than the device itself, but by patients with active intervention to gain momentum (eg: rely on gravity as a power device). |
Share