1
/
of
7
PayPal, credit cards. Download editable-PDF and invoice in 1 second!
YS/T 575.23-2009 English PDF (YS/T575.23-2009)
YS/T 575.23-2009 English PDF (YS/T575.23-2009)
Regular price
$150.00
Regular price
Sale price
$150.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YS/T 575.23-2009: Method for chemical analysis of aluminum ores. Part 23: Determination of element contents X-ray fluorescence spectrometric mehtod
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YS/T 575.23-2009 (Self-service in 1-minute)
Newer / historical versions: YS/T 575.23-2009
Preview True-PDF
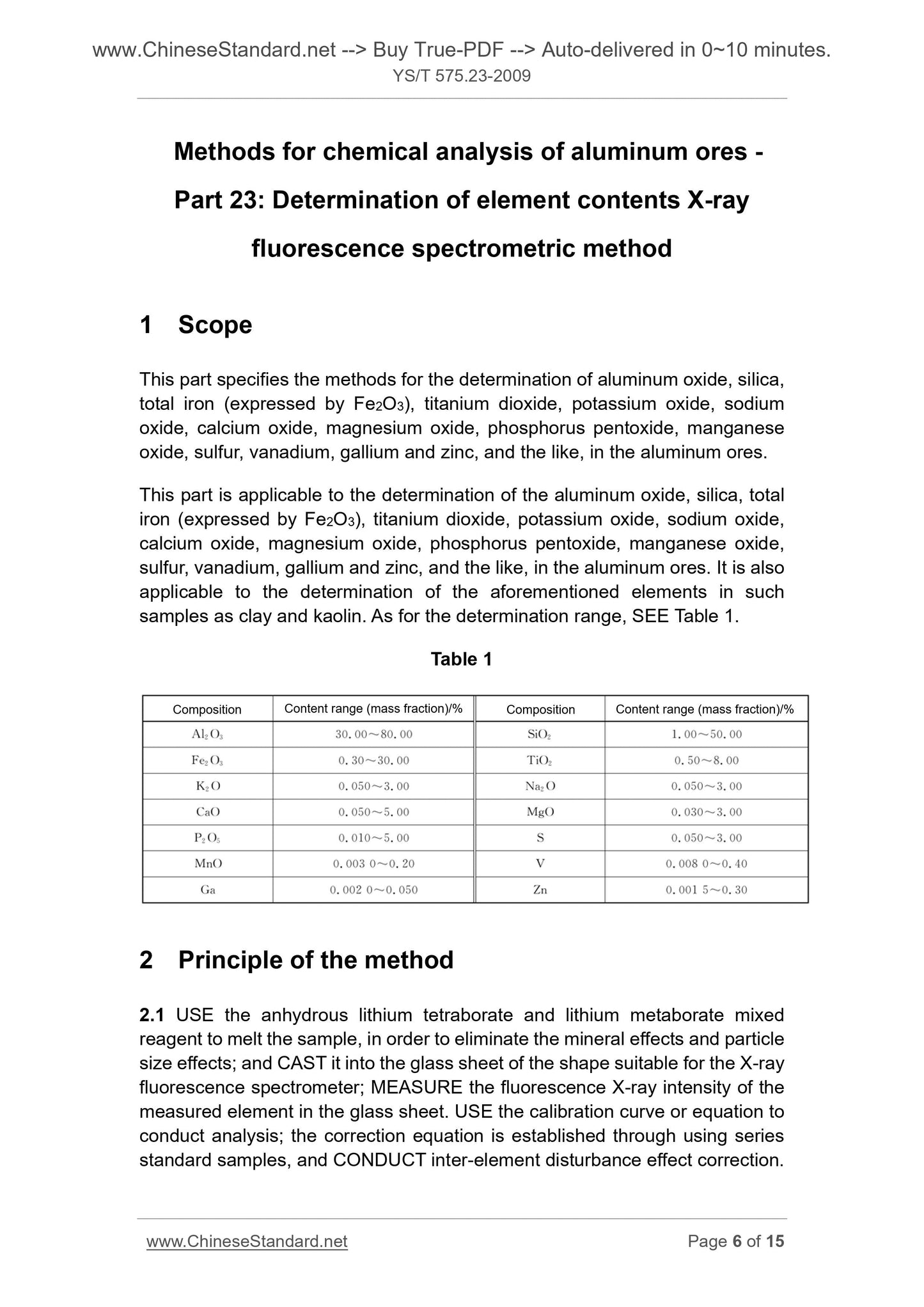
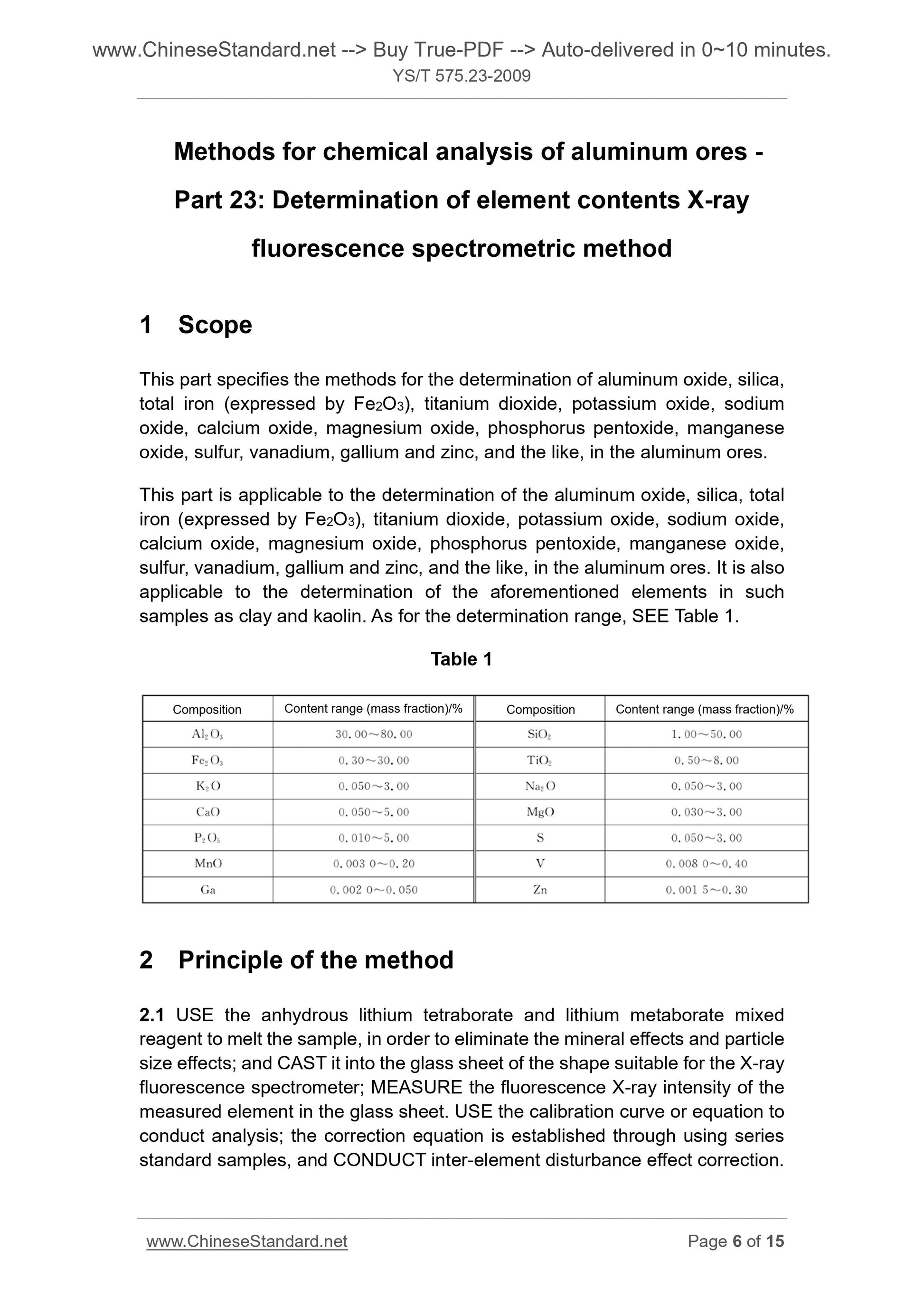
Scope
This part specifies the methods for the determination of aluminum oxide, silica,total iron (expressed by Fe2O3), titanium dioxide, potassium oxide, sodium
oxide, calcium oxide, magnesium oxide, phosphorus pentoxide, manganese
oxide, sulfur, vanadium, gallium and zinc, and the like, in the aluminum ores.
This part is applicable to the determination of the aluminum oxide, silica, total
iron (expressed by Fe2O3), titanium dioxide, potassium oxide, sodium oxide,
calcium oxide, magnesium oxide, phosphorus pentoxide, manganese oxide,
sulfur, vanadium, gallium and zinc, and the like, in the aluminum ores. It is also
applicable to the determination of the aforementioned elements in such
samples as clay and kaolin. As for the determination range, SEE Table 1.
Table 1
Basic Data
| Standard ID | YS/T 575.23-2009 (YS/T575.23-2009) |
| Description (Translated English) | Method for chemical analysis of aluminum ores. Part 23: Determination of element contents X-ray fluorescence spectrometric mehtod |
| Sector / Industry | Nonferrous Metallurgy Industry Standard (Recommended) |
| Classification of Chinese Standard | H30 |
| Classification of International Standard | 77.120.10 |
| Word Count Estimation | 9,930 |
| Date of Issue | 2009-12-04 |
| Date of Implementation | 2010-06-01 |
| Adopted Standard | AS 2564-1982, MOD |
| Regulation (derived from) | MIIT [2009] No. 66 |
| Issuing agency(ies) | Ministry of Industry and Information Technology |
| Summary | This standard specifies the X-ray fluorescence spectrometry element content of bauxite method. This standard applies in bauxite aluminum oxide, silica, total iron (expressed in Fe2O3), titanium dioxide, potassium hydroxide, sodium oxide, calcium oxide, magnesium oxide, phosphorus pentoxide, manganese oxide, sulfur, vanadium, gallium, and zinc determination. Also applies to clay, kaolin, etc. Determination of the composition of the sample. |
Share