1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
QC/T 1131-2020 English PDF (QC/T1131-2020)
QC/T 1131-2020 English PDF (QC/T1131-2020)
Regular price
$380.00
Regular price
Sale price
$380.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
QC/T 1131-2020: Methods of Detecting Polycyclic Aromatic Hydrocarbons in Automotive Materials
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click QC/T 1131-2020 (Self-service in 1-minute)
Newer / historical versions: QC/T 1131-2020
Preview True-PDF
Scope
This Document specifies the terms and definitions of the methods of detectingpolycyclic aromatic hydrocarbons in automotive materials, as well as the detection
methods of gas chromatography-mass spectrometry, high performance liquid
chromatography, proton nuclear magnetic resonance spectroscopy, and the like
contents.
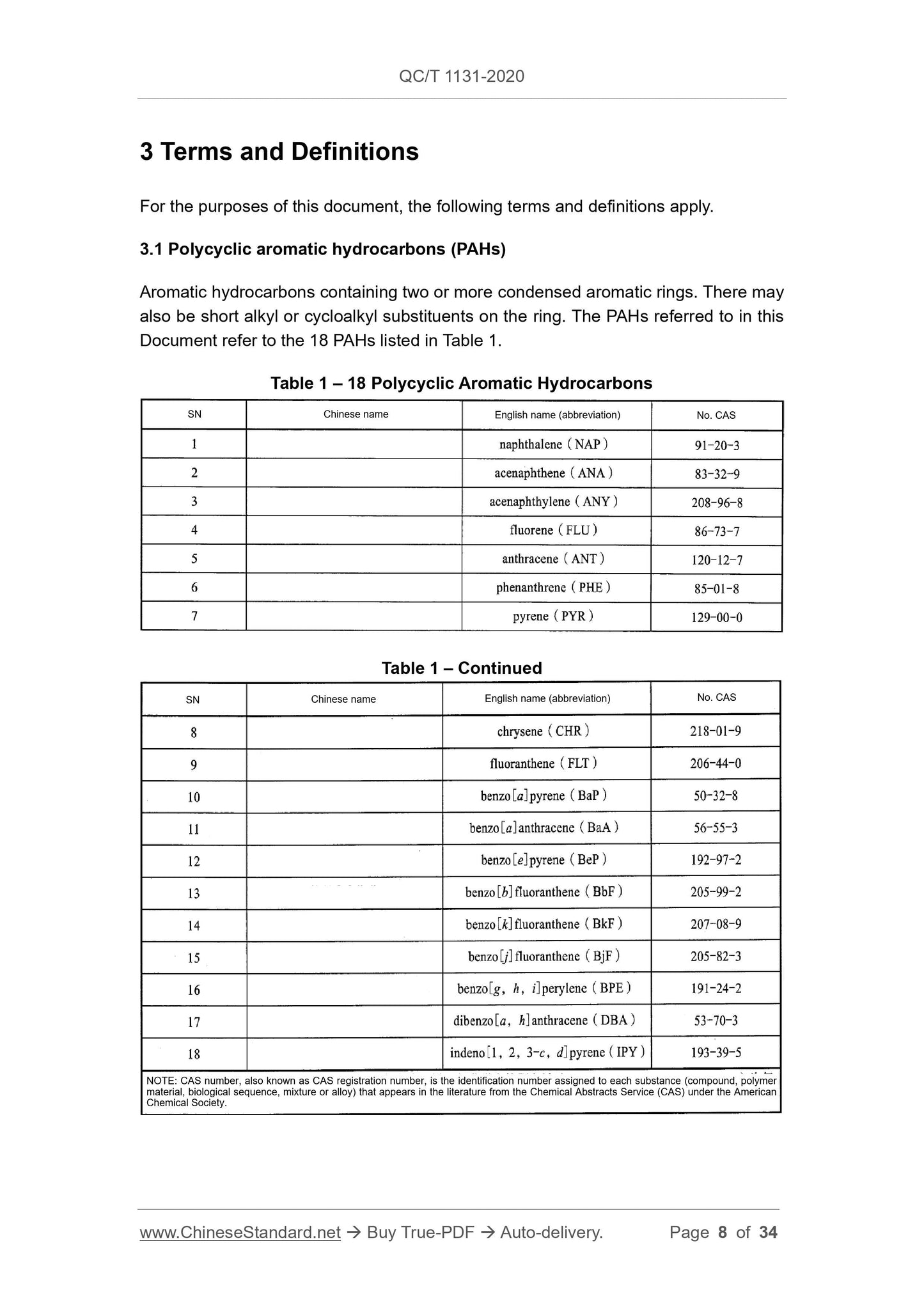
This Document is applicable to the qualitative and quantitative testing of 18 polycyclic
aromatic hydrocarbons in automotive materials. Among them, gas chromatography-
mass spectrometry is suitable for testing polycyclic aromatic hydrocarbons in textiles,
plastics, thermoplastic elastomers, rubber and leather materials; high performance
liquid chromatography is suitable for testing polycyclic aromatic hydrocarbons in
plastics and rubber materials; proton nuclear magnetic resonance spectroscopy is
suitable for testing polycyclic aromatic hydrocarbons in rubber materials.
Basic Data
| Standard ID | QC/T 1131-2020 (QC/T1131-2020) |
| Description (Translated English) | Methods of Detecting Polycyclic Aromatic Hydrocarbons in Automotive Materials |
| Sector / Industry | Automobile and Vehicle Industry Standard (Recommended) |
| Classification of Chinese Standard | T40 |
| Word Count Estimation | 25,254 |
| Date of Issue | 2020-08-31 |
| Date of Implementation | 2021-01-01 |
| Regulation (derived from) | Ministry of Industry and Information Technology Announcement No. 37 (2020) |
| Issuing agency(ies) | Ministry of Industry and Information Technology |
Share