1
/
of
8
PayPal, credit cards. Download editable-PDF and invoice in 1 second!
GB/T 14849.3-2007 English PDF (GB/T14849.3-2007)
GB/T 14849.3-2007 English PDF (GB/T14849.3-2007)
Regular price
$140.00
Regular price
Sale price
$140.00
Unit price
/
per
Shipping calculated at checkout.
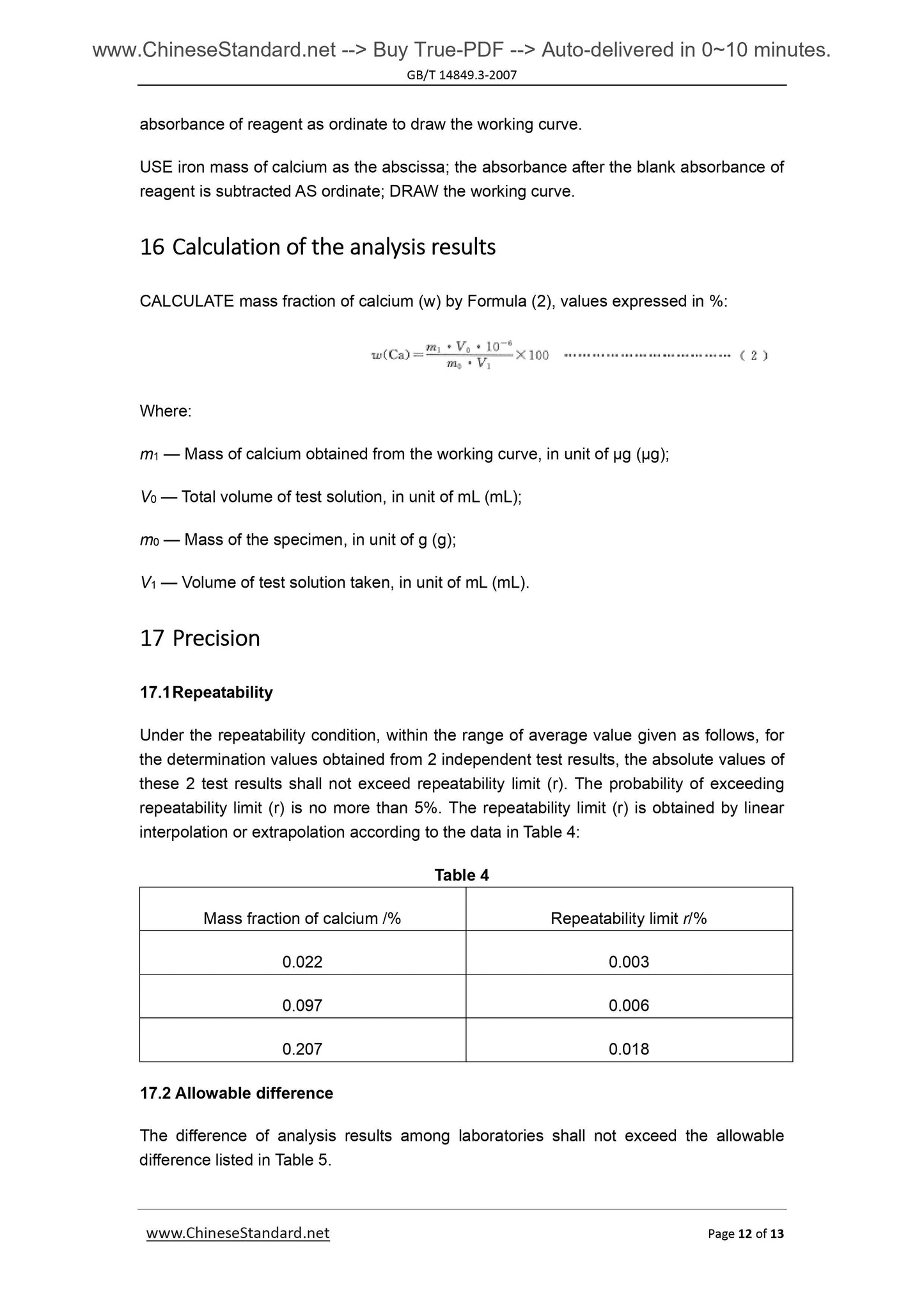
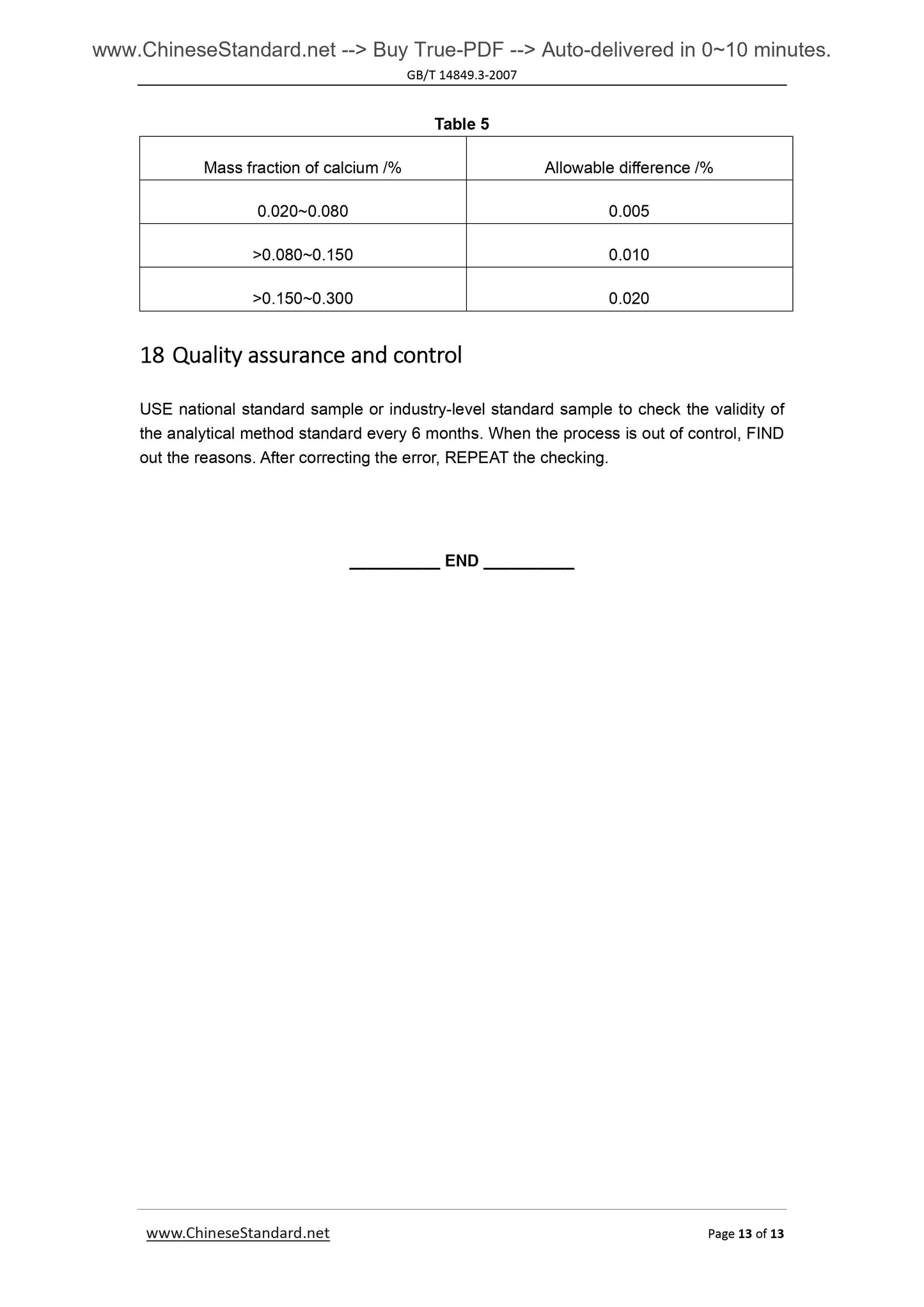
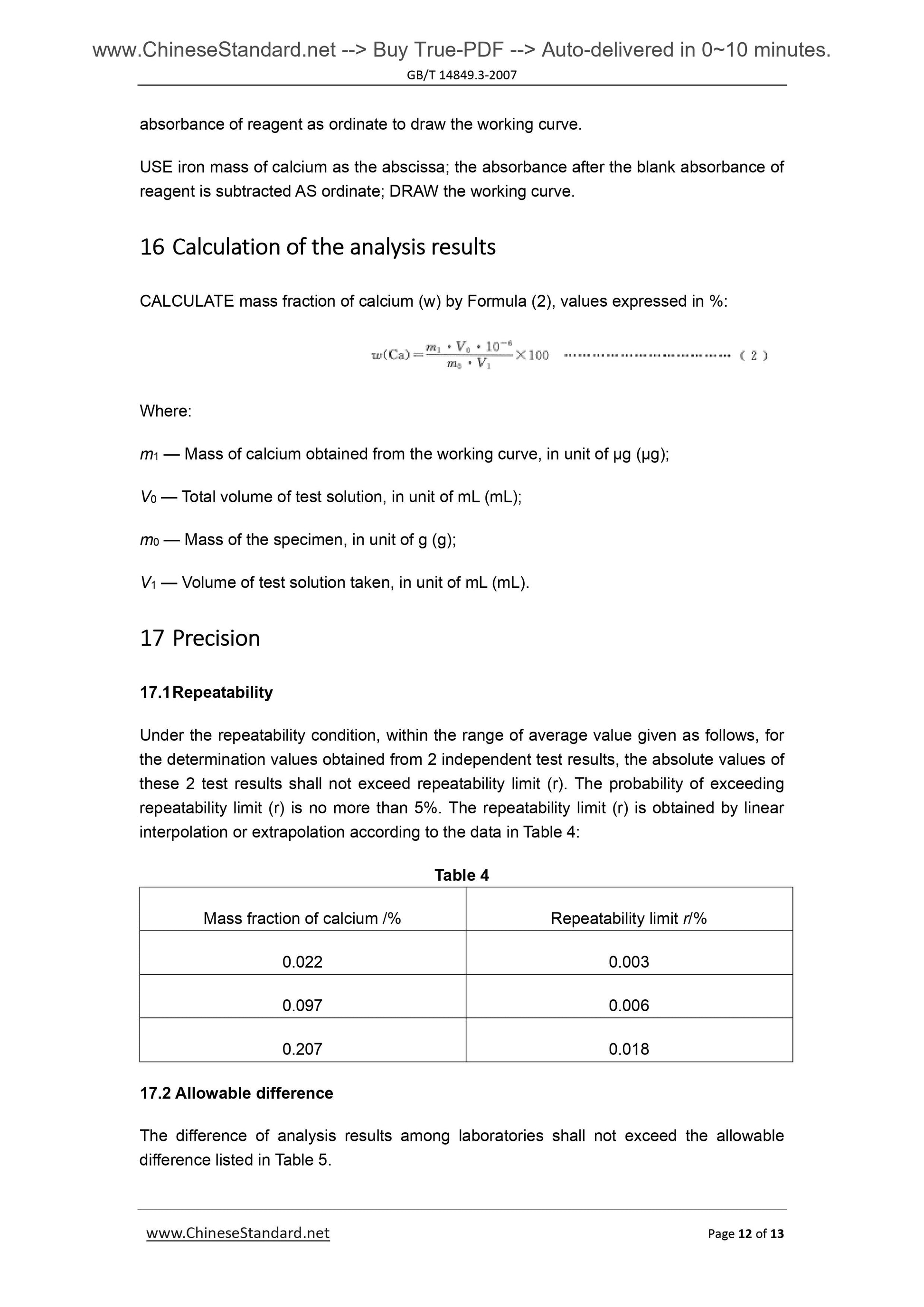
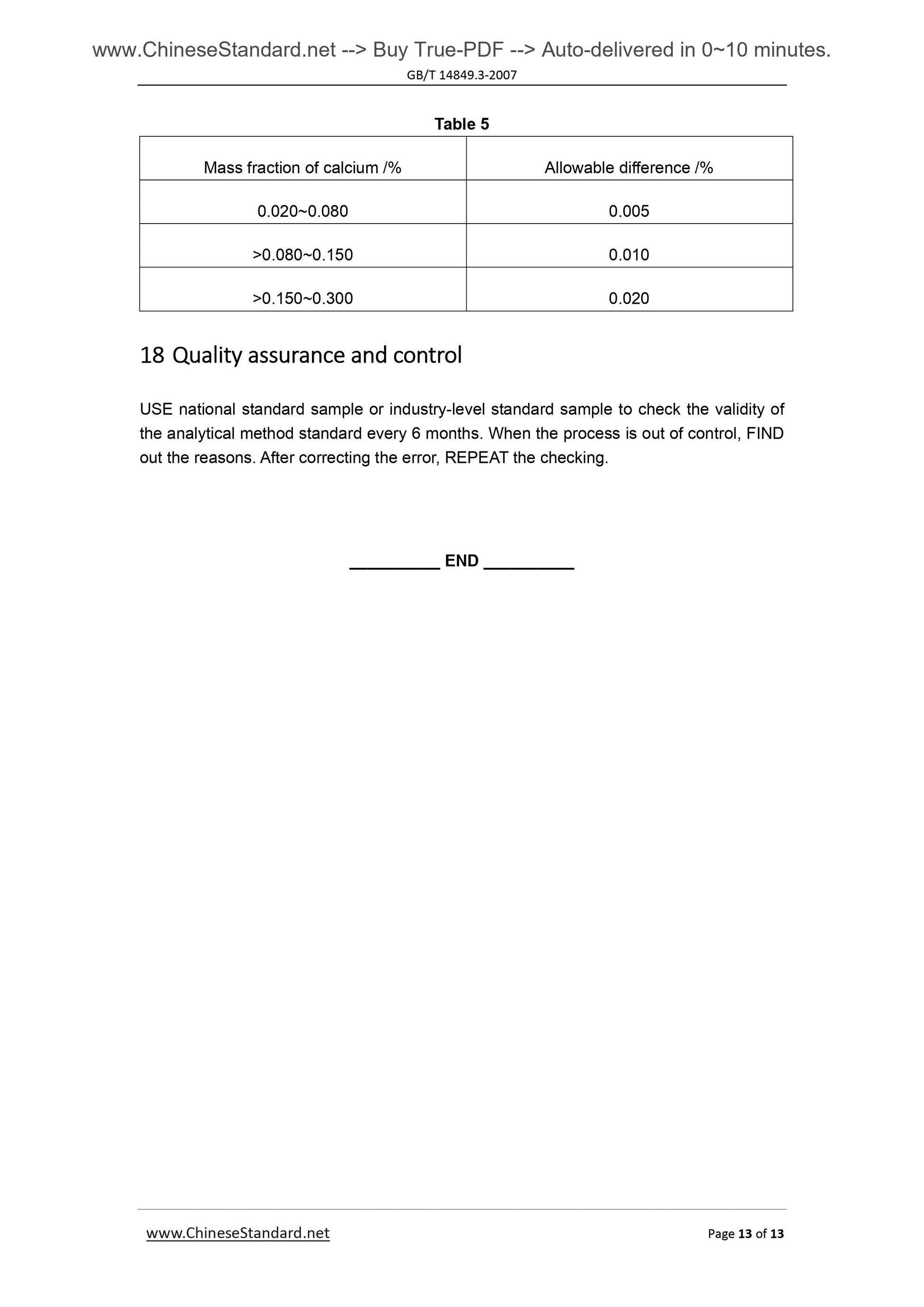
Couldn't load pickup availability
GB/T 14849.3-2007: Industrial silicon chemical analysis -- Part 3: Determination of calcium content
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click GB/T 14849.3-2007 (Self-service in 1-minute)
Newer / historical versions: GB/T 14849.3-2007
Preview True-PDF
Scope
This Method specifies the method for determination of calcium content in silicon metal.This Method applies to the determination of calcium content in silicon metal.
Determination range (mass fraction). 0.020%~0.30%.
2 Method summary
The specimen is decomposed with hydrofluoric acid and nitric acid. Perchloric acid’s
smoking removes silicon, fluorine, etc. And the residue is dissolved with hydrochloric acid.
Lanthanum salt is used to control the interference of aluminum. Determine the
absorbance of calcium by air-acetylene flame, at flame atomic absorption spectrometry
wavelength of 422.7nm.
Basic Data
| Standard ID | GB/T 14849.3-2007 (GB/T14849.3-2007) |
| Description (Translated English) | Industrial silicon chemical analysis -- Part 3: Determination of calcium content |
| Sector / Industry | National Standard (Recommended) |
| Classification of Chinese Standard | H12 |
| Classification of International Standard | 77.120.10 |
| Word Count Estimation | 8,899 |
| Date of Implementation | 2008-04-01 |
| Older Standard (superseded by this standard) | GB/T 14849.3-1993 |
| Regulation (derived from) | China National Standard Approval Announcement2007 No.12 (Total No.112) |
| Issuing agency(ies) | General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China |
| Summary | This standard specifies the determination of the calcium content of the silicon industry. |
Share