1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
GB 5009.267-2020 English PDF
GB 5009.267-2020 English PDF
Regular price
$205.00
Regular price
Sale price
$205.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
GB 5009.267-2020: National food safety standard - Determination of iodine in foods
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click GB 5009.267-2020 (Self-service in 1-minute)
Newer / historical versions: GB 5009.267-2020
Preview True-PDF
Scope
This standard specifies the determination method of iodine content in food.The method 1 Inductively coupled plasma mass spectrometry is suitable for the
determination of iodine in food.
The method 2 Redox titration is suitable for the determination of iodine in algae
and its products.
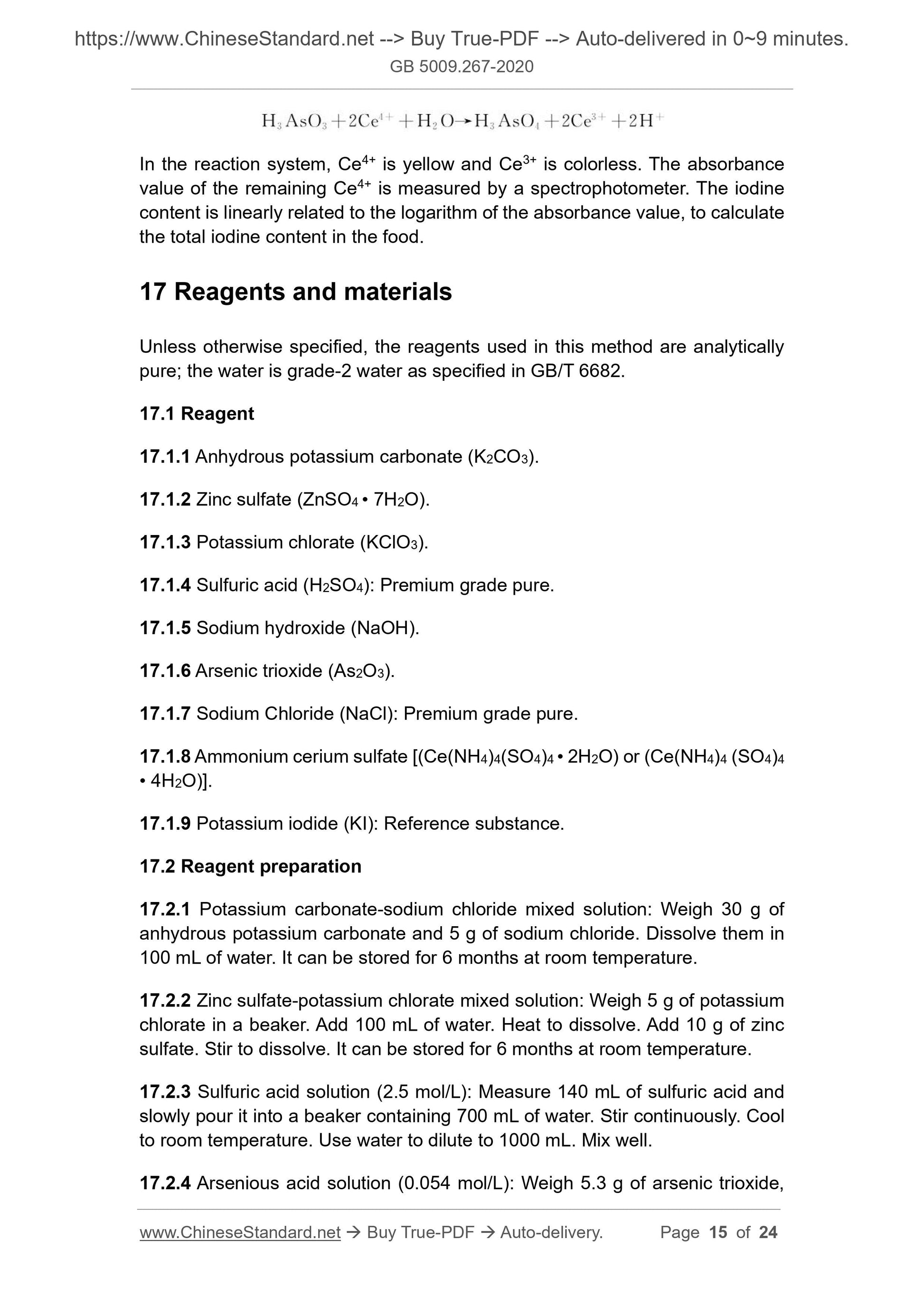
The method 3 Arsenic-cerium catalytic spectrophotometry is suitable for the
determination of iodine in food, vegetables, fruits, beans and their products,
milk and their products, meat, fish and eggs.
The method 4 Gas chromatography is applicable to the determination of
nutritional fortifier iodine in infant formula foods and dairy products (except
infant formula foods for special medical purposes and formula foods for special
medical purposes).
Method 1 -- Inductively coupled plasma mass
spectrometry (ICP-MS)
Basic Data
| Standard ID | GB 5009.267-2020 (GB5009.267-2020) |
| Description (Translated English) | National food safety standard - Determination of iodine in foods |
| Sector / Industry | National Standard |
| Classification of Chinese Standard | X09 |
| Word Count Estimation | 16,176 |
| Date of Issue | 2020-09-11 |
| Date of Implementation | 2021-03-11 |
| Older Standard (superseded by this standard) | GB 5009.267-2016; SN/T 3154-2012 |
| Regulation (derived from) | National Health Commission Announcement No. 7 (2020) of the State Administration for Market Regulation |
| Issuing agency(ies) | National Health Commission of the People's Republic of China, State Administration for Market Regulation |
Share