1
/
of
6
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
GB 1903.47-2020 English PDF
GB 1903.47-2020 English PDF
Regular price
$115.00
Regular price
Sale price
$115.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
GB 1903.47-2020: National food safety standard - Food Nutritional Fortification Substance - Ferrous Lactate
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click GB 1903.47-2020 (Self-service in 1-minute)
Newer / historical versions: GB 1903.47-2020
Preview True-PDF
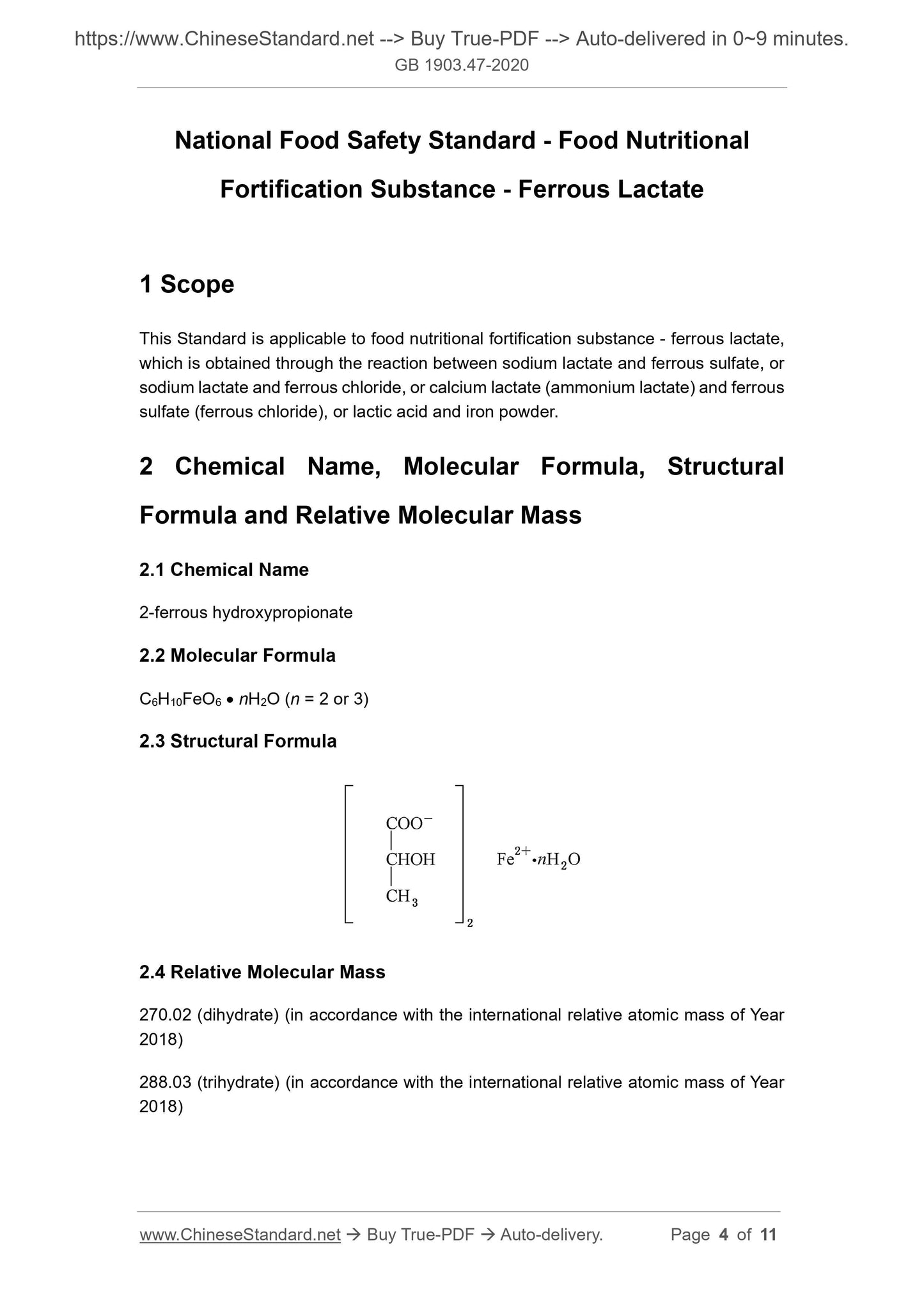
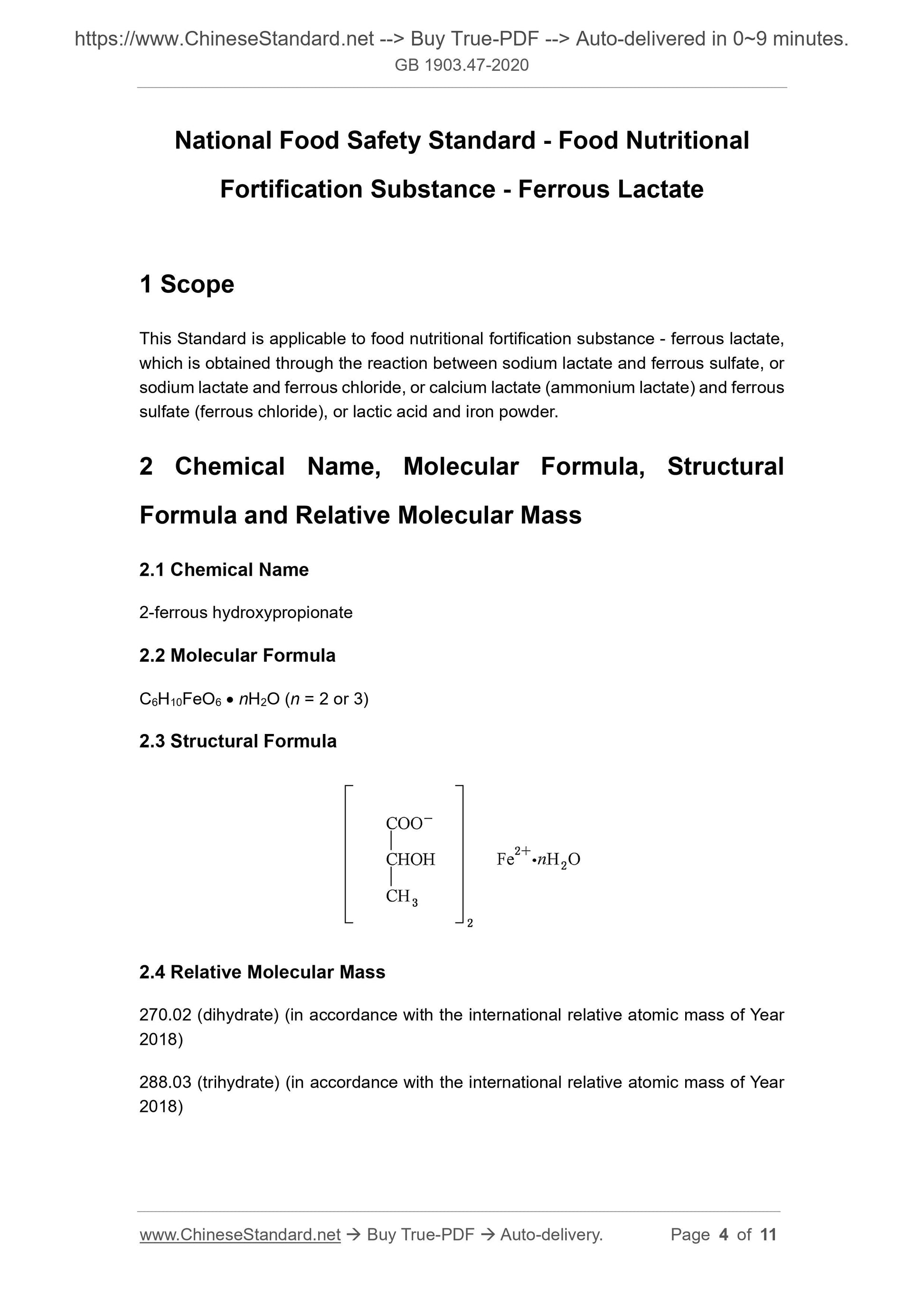
Scope
This Standard is applicable to food nutritional fortification substance - ferrous lactate,which is obtained through the reaction between sodium lactate and ferrous sulfate, or
sodium lactate and ferrous chloride, or calcium lactate (ammonium lactate) and ferrous
sulfate (ferrous chloride), or lactic acid and iron powder.
2 Chemical Name, Molecular Formula, Structural
Formula and Relative Molecular Mass
2.1 Chemical Name
2-ferrous hydroxypropionate
2.2 Molecular Formula
C6H10FeO6 nH2O (n = 2 or 3)
2.3 Structural Formula
2.4 Relative Molecular Mass
270.02 (dihydrate) (in accordance with the international relative atomic mass of Year
2018)
288.03 (trihydrate) (in accordance with the international relative atomic mass of Year
2018)
Basic Data
| Standard ID | GB 1903.47-2020 (GB1903.47-2020) |
| Description (Translated English) | National food safety standard - Food Nutritional Fortification Substance - Ferrous Lactate |
| Sector / Industry | National Standard |
| Classification of Chinese Standard | X09 |
| Word Count Estimation | 9,986 |
| Date of Issue | 2020-09-11 |
| Date of Implementation | 2021-03-11 |
| Older Standard (superseded by this standard) | GB 6781-2007 |
| Regulation (derived from) | National Health Commission Announcement No. 7 (2020) of the State Administration for Market Regulation |
| Issuing agency(ies) | National Health Commission of the People's Republic of China, State Administration for Market Regulation |
Share