1
/
of
7
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
GB 1903.44-2020 English PDF
GB 1903.44-2020 English PDF
Regular price
$155.00
Regular price
Sale price
$155.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
GB 1903.44-2020: National food safety standard - Food Nutritional Fortification Substance - Hydroxocobalamin
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click GB 1903.44-2020 (Self-service in 1-minute)
Newer / historical versions: GB 1903.44-2020
Preview True-PDF
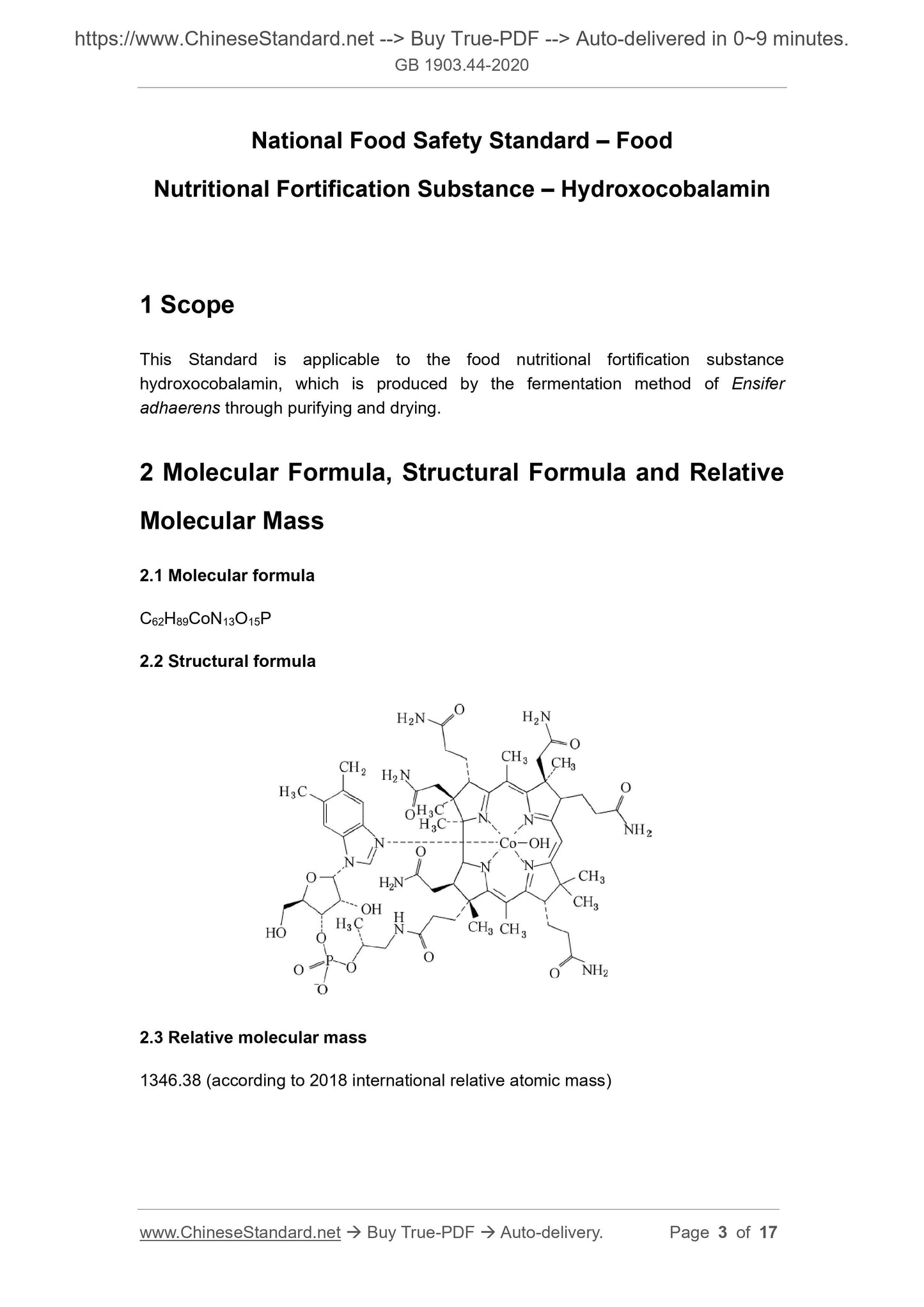
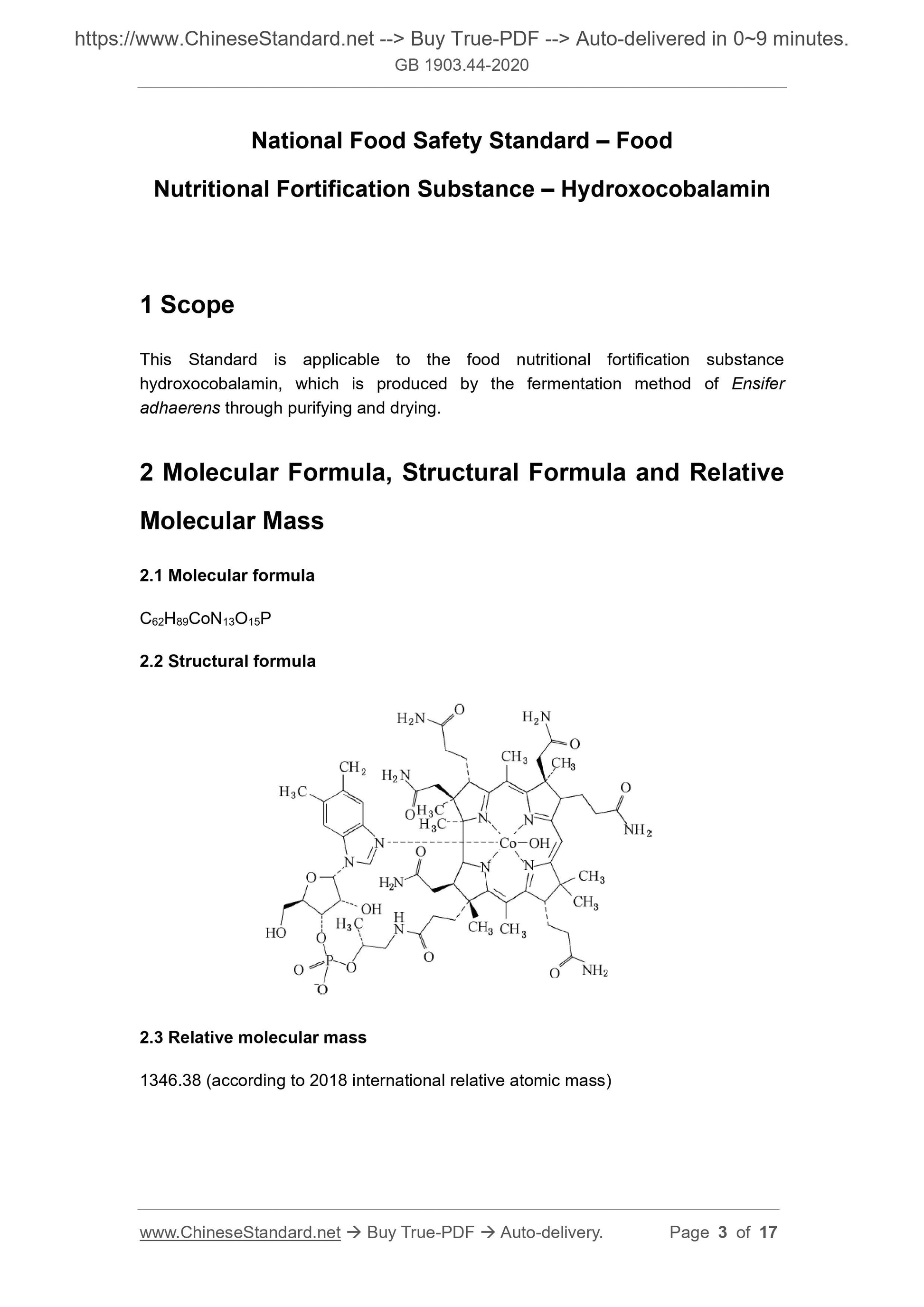
Scope
This Standard is applicable to the food nutritional fortification substancehydroxocobalamin, which is produced by the fermentation method of Ensifer
adhaerens through purifying and drying.
Basic Data
| Standard ID | GB 1903.44-2020 (GB1903.44-2020) |
| Description (Translated English) | National food safety standard - Food Nutritional Fortification Substance - Hydroxocobalamin |
| Sector / Industry | National Standard |
| Classification of Chinese Standard | X09 |
| Word Count Estimation | 12,140 |
| Date of Issue | 2020-09-11 |
| Date of Implementation | 2021-03-11 |
| Regulation (derived from) | National Health Commission Announcement No. 7 (2020) of the State Administration for Market Regulation |
| Issuing agency(ies) | National Health Commission of the People's Republic of China, State Administration for Market Regulation |
Share