1
/
of
8
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
GB 1886.324-2021 English PDF
GB 1886.324-2021 English PDF
Regular price
$95.00
Regular price
Sale price
$95.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
GB 1886.324-2021: National food safety standard - Food additives - Metatartaric acid
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click GB 1886.324-2021 (Self-service in 1-minute)
Newer / historical versions: GB 1886.324-2021
Preview True-PDF
Scope
This Standard applies to the food additive metatartaric acid which is obtained,with tartaric acid as the raw material, by baking at 168 °C ~ 172 °C for 3.5 h, or
heating to 180 °C ~ 190 °C, and then naturally cooled.
2 Chemical name, molecular formula, structural
formula and relative molecular mass
2.1 Chemical name
Metatartaric acid
2.2 Molecular formula
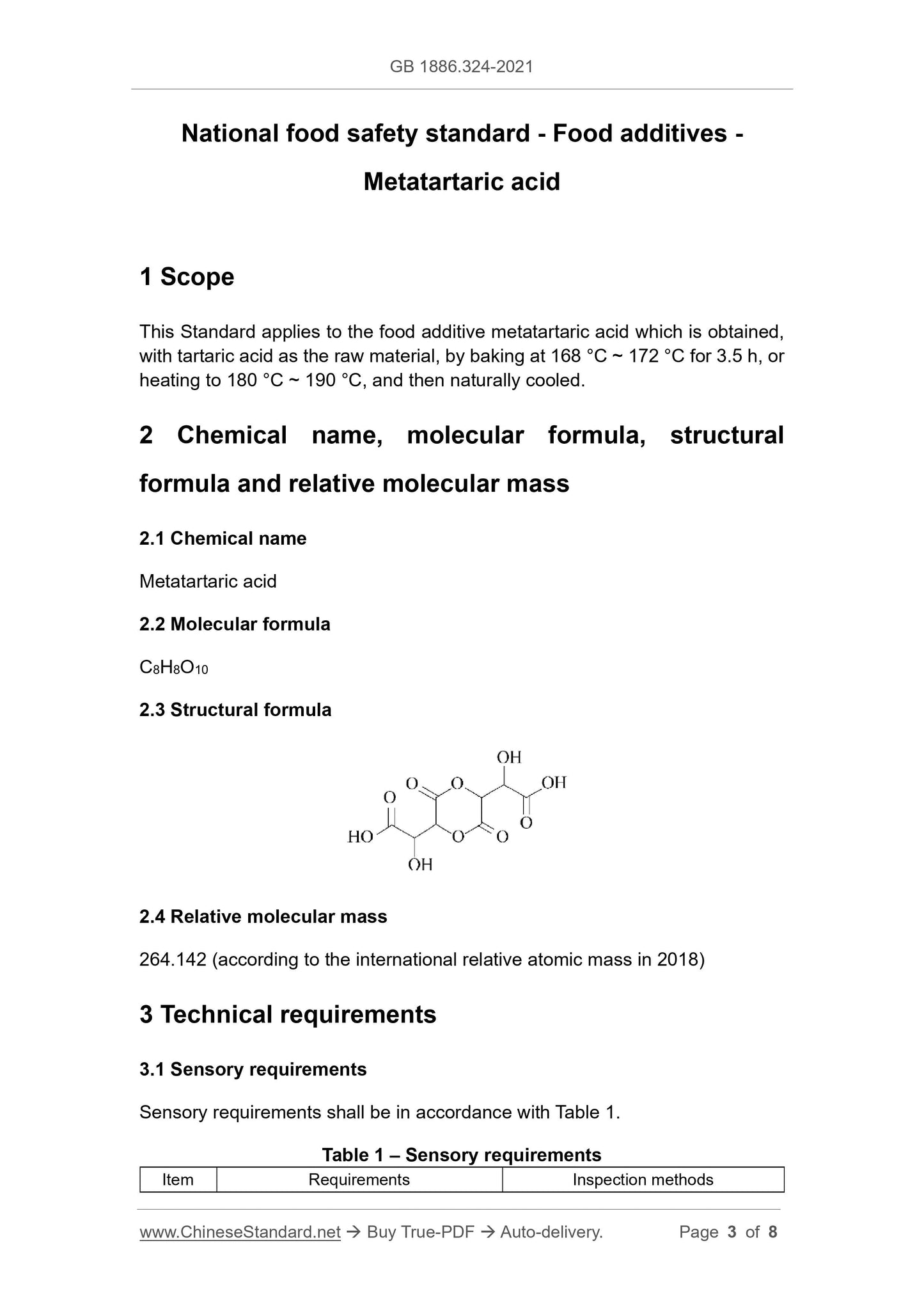
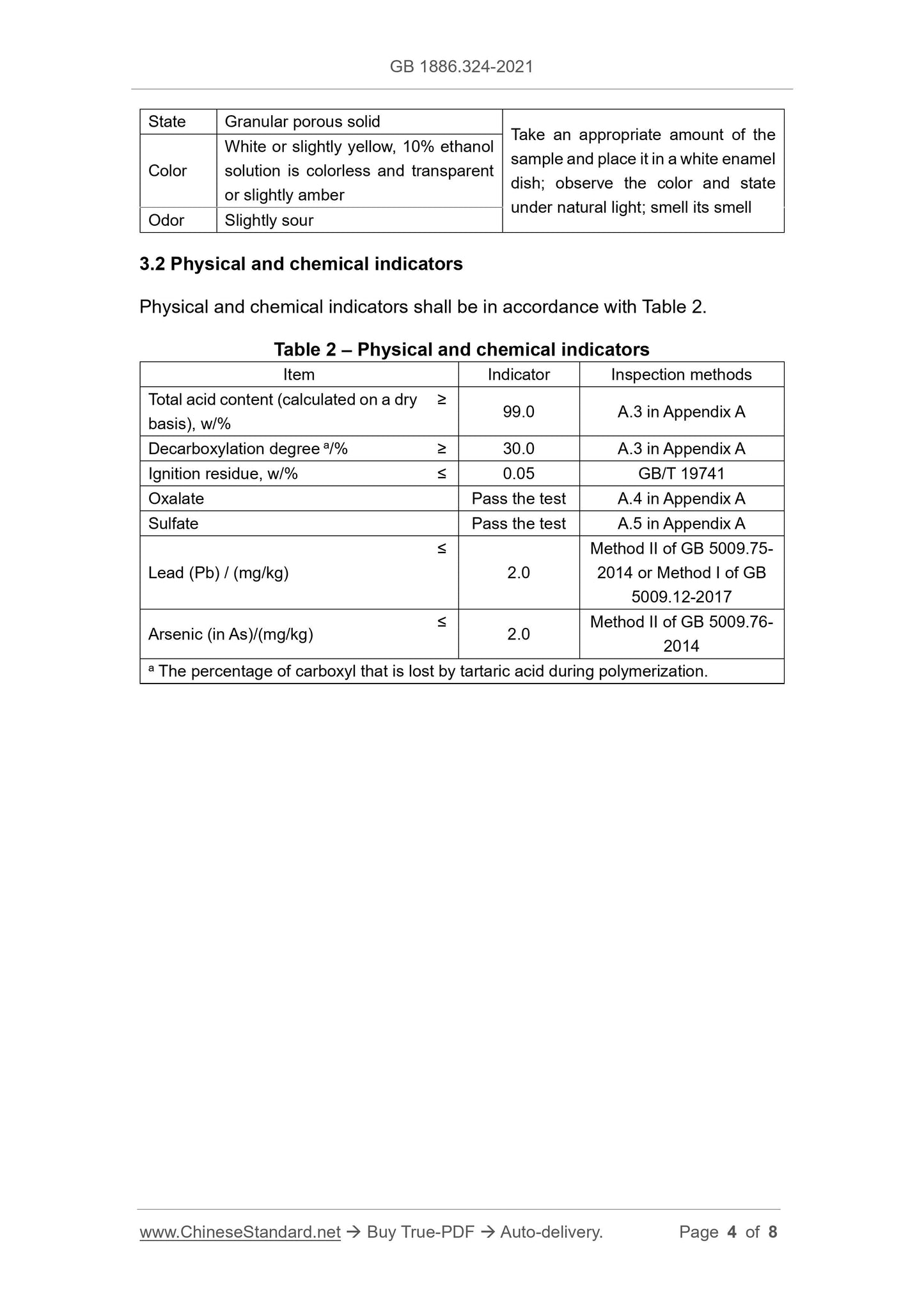
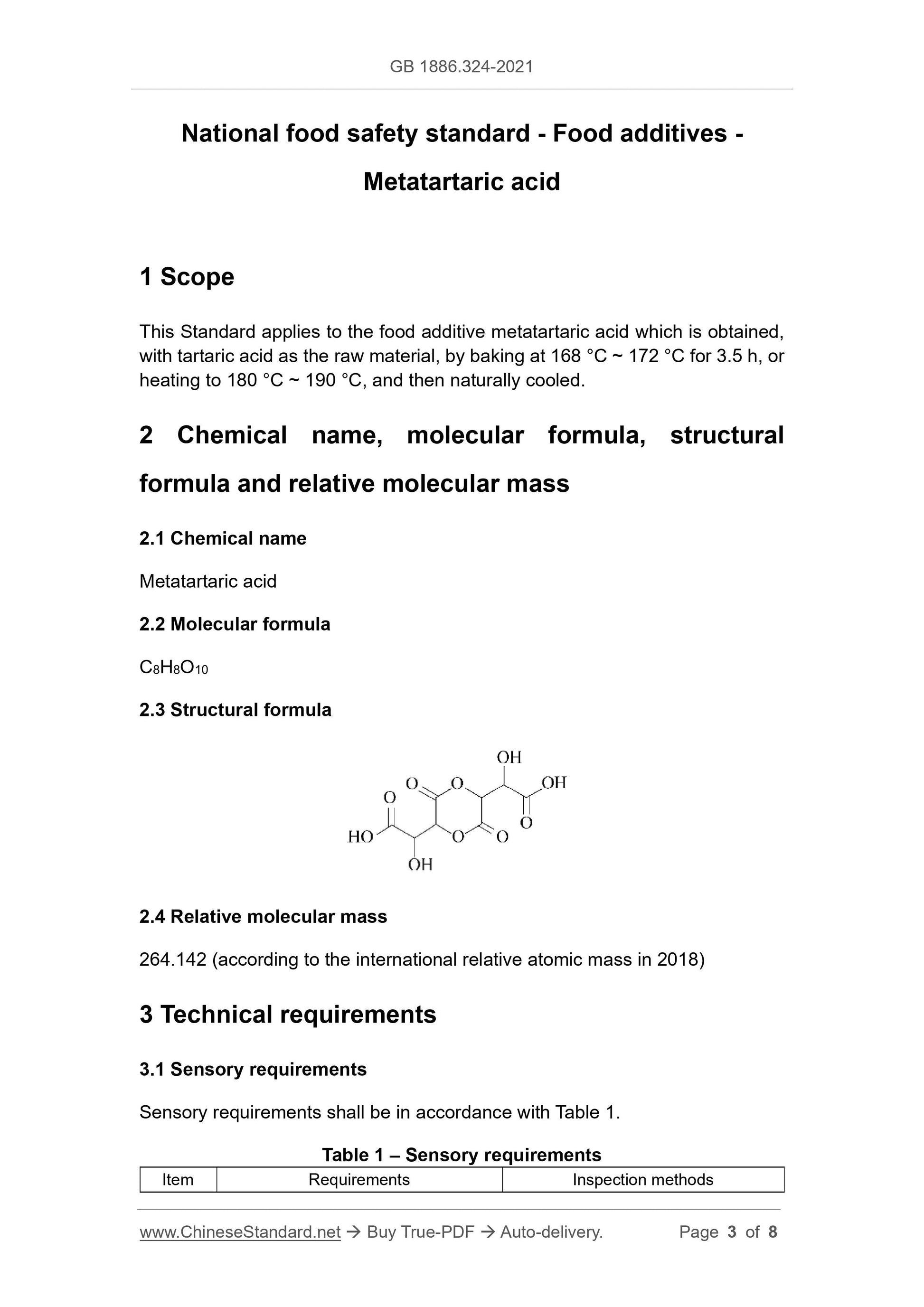
C8H8O10
2.3 Structural formula
2.4 Relative molecular mass
264.142 (according to the international relative atomic mass in 2018)
Basic Data
| Standard ID | GB 1886.324-2021 (GB1886.324-2021) |
| Description (Translated English) | National food safety standard - Food additives - Metatartaric acid |
| Sector / Industry | National Standard |
| Classification of Chinese Standard | X09 |
| Word Count Estimation | 7,791 |
| Issuing agency(ies) | National Health Commission of the People's Republic of China, State Administration for Market Regulation |
Share