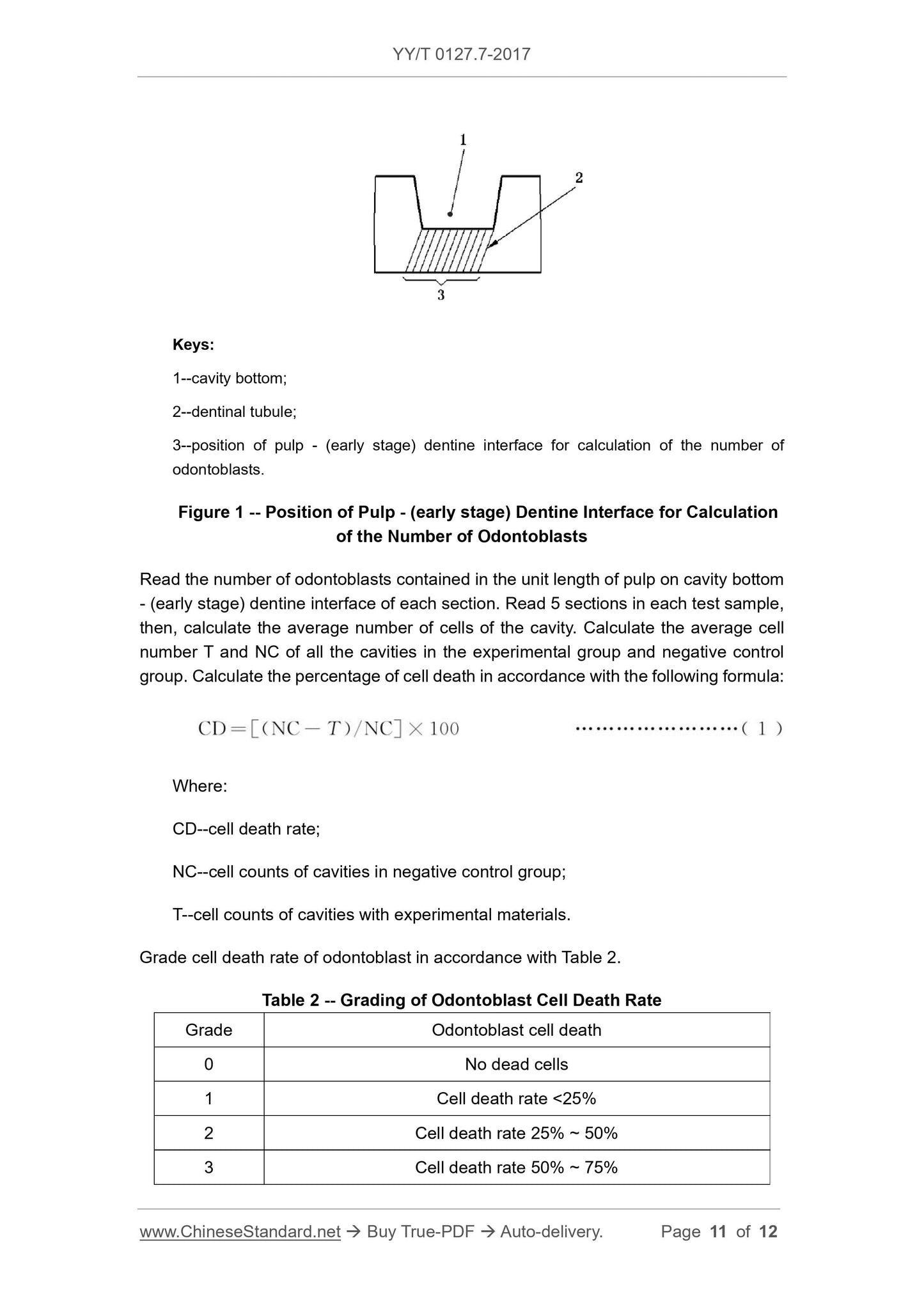
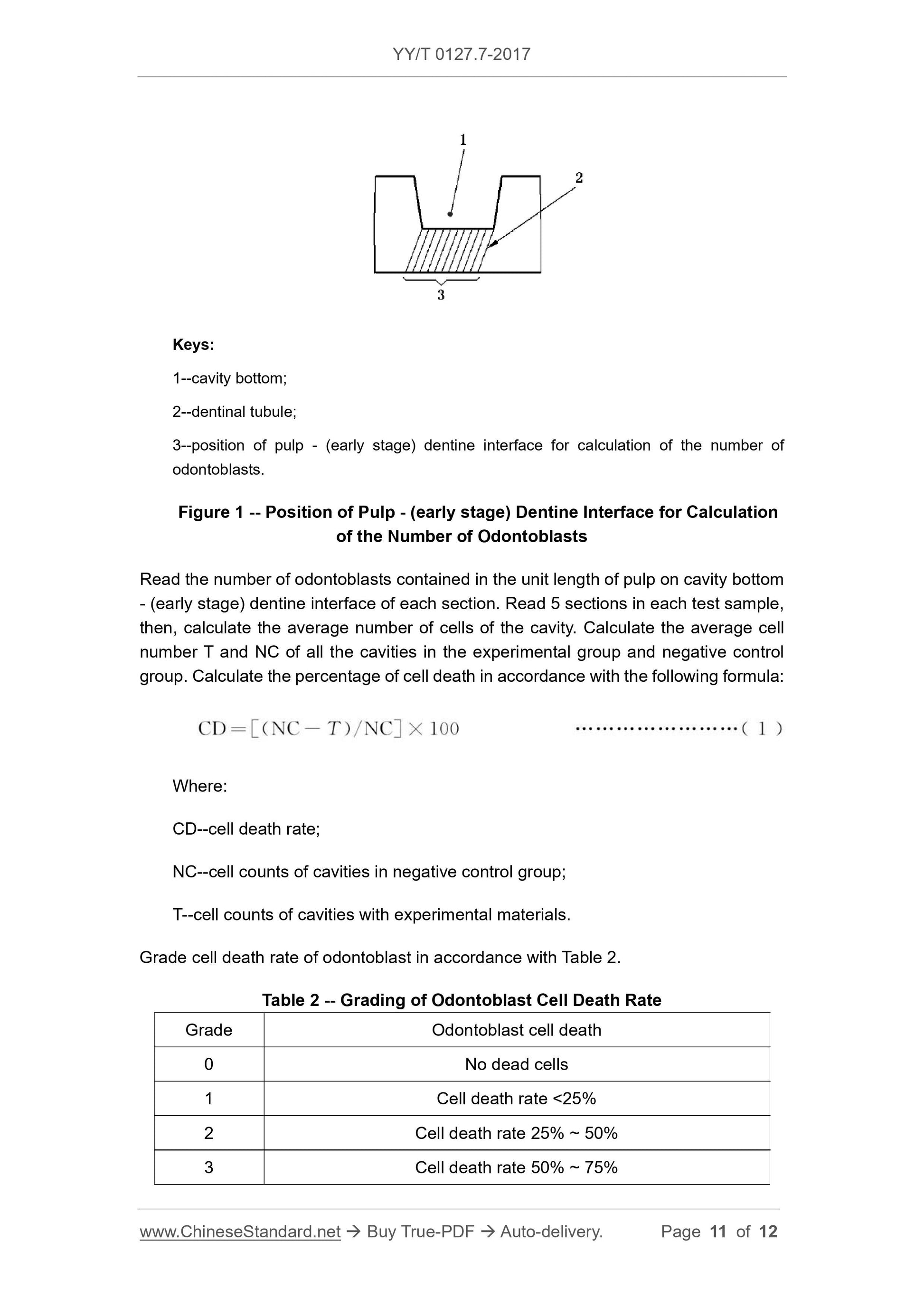
1
/
of
12
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY/T 0127.7-2017 English PDF (YY/T0127.7-2017)
YY/T 0127.7-2017 English PDF (YY/T0127.7-2017)
Regular price
$130.00
Regular price
Sale price
$130.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY/T 0127.7-2017: Biological evaluation of dental materials -- Part 2: Biological evaluation test method of dental materials -- Pulp and dentine usage test
Delivery: 9 seconds. Download (and Email) true-PDF + Invoice.Get Quotation: Click YY/T 0127.7-2017 (Self-service in 1-minute)
Newer / historical versions: YY/T 0127.7-2017
Preview True-PDF
Scope
This part of YY/T 0127 specifies methods of dental materials, pulp and dentine usagetest.
This part shall be applied to evaluate biocompatibility of dental materials, dentine and
endodontic pulp, including evaluation methods and steps that are indispensable to the
anticipated clinical application of the materials.
Basic Data
| Standard ID | YY/T 0127.7-2017 (YY/T0127.7-2017) |
| Description (Translated English) | Biological evaluation of dental materials -- Part 2: Biological evaluation test method of dental materials -- Pulp and dentine usage test |
| Sector / Industry | Medical Device and Pharmaceutical Industry Standard (Recommended) |
| Classification of Chinese Standard | C33 |
| Classification of International Standard | 11.060.10 |
| Word Count Estimation | 8,846 |
| Date of Issue | 2017-03-28 |
| Date of Implementation | 2018-04-01 |
| Older Standard (superseded by this standard) | YY/T 0127.7-2001 |
| Regulation (derived from) | China Food and Drug Administration Announcement 2017 No. 38 |
| Issuing agency(ies) | State Food and Drug Administration |
Share